Lab 8 - Metabolism
advertisement

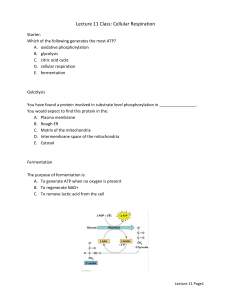
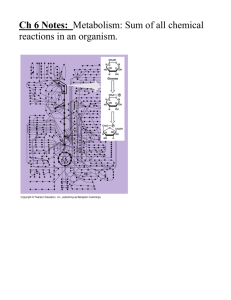
Plant Science 1203L Laboratory 11 - Plant Metabolism Name: Today’s lab is on plant metabolism. So possibly the best place to start out with is a definition of the term. Metabolism is the chemical and physical process continuously going on in living organisms and cells, consisting of anabolism and catabolism. Anabolism is the process by which food is changed into living tissue. Since plants are autotrophic (able to produce their own food) the definition of anabolism also covers the production of food through photosynthesis, something that animals are incapable of doing. We will be studying photosynthesis next lab. Catabolism is the process by which food is converted into energy and wastes of a simpler chemical composition. For this lab we will be concentrating on the catabolic form of metabolism. It is obvious that metabolism covers all the biochemistry that is occurring within plant cells. Consequently, we will only touch on the major catabolic pathways in the plant. These processes that provide energy are glycolysis, the krebs cycle (tricarboxylic cycle or citric acid cycle) and finally oxidative phosphorylation (terminal oxidation). Enzymes - Virtually all the steps in the metabolism that we will talk about are driven by these enzymes. Some steps in metabolism may require more than one substrate (compound) in order to accomplish it’s role as a catalyst. In energy producing catabolism certain compounds (co-factors) in addition to the substrate are particularly important. These are ATP, ADP, NADH, NAD+, FADH2 and phosphate (P). ATP has been called the energy currency of the cell and is involved in numerous enzyme mediated reactions in the cell. Starch - is the carbohydrate storage compound we have seen numerous times during lab this semester. Before it can be used as a substrate for energy production it must be digested or broken down into its’ basic building blocks of simple sugars such as glucose. Again this process is also controlled by enzymes (amylase). Since starch is a storage food reserve for the corn embryo it must be broken down to free sugars which will provide energy to the embryo through catabolism of the sugars. As the starch grains are digested they will become smaller and stain less strongly with the iodine solution. Exercise 1. Examine microscope 1 and observe normal starch grains from corn that have been stained with iodine. Compare what you see under this microscope with that of microscope 2, which has starch grains from corn that has been germinated. Answer the following questions: Are more of the starch grains from the germinated corn smaller than those from the ungerminated corn? Are some of the starch grains from germinated corn less stained than from the ungerminated corn? We have observed the first step in utilization of storage starch for use by the growing embryo. The second step will start to extract the energy from the free sugars as high energy compounds (ATP, NADH) that the plant can use in other biochemical processes. Glycolysis - Below is a diagrammatic illustration of glycolysis. This process is the first step in the extraction of energy from carbohydrate. In order to get the process started energy in the form of ATP must first be used. Later this ATP will be recovered along with 2 additional ATP for each molecule of sugar catabolized. Glycolysis occurs in the cell cytoplasm and not within any of the organelles. Two pyruvate molecules are produced for each glucose molecule as the end product of glycolysis. It represents an intermediate step in energy production in the cell. If oxygen is available then pyruvate can be further degraded to yield much more energy (aerobic respiration). If oxygen is not available and the cell can undergo anaerobic respiration, then pyruvate will be converted to a end product such as ethanol or lactic acid (anaerobic respiration. The diagrams above show the chemistry of this conversion. Note that in alcoholic fermentation CO2 is produced, but not in lactic acid fermentation. Moreover, note that in converting pyruvate to either lactate or ethanol NADH is used and NAD+ is regenerated. This NAD+ is required in the glycolytic pathway during the step from glyceraldehyde-3-phosphate to 1,3-biphosphoglycerate (see glycolysis diagram above). Without this regeneration of NAD+ the glycolytic pathway would stop! Exercise 2 . On demonstration is a mixture of barley malt, sugar, hops and yeast. The mixture was placed into a flask and an airlock attached so that no oxygen is available to the yeast. Note the bubbling of the airlock. Answer the following questions: What gas is being released? What catabolic stage of energy metabolism does this represent? Is this an efficient process for obtaining energy? Why? Krebs cycle - The next step in energy metabolism occurs when the pyruvate is moved into the mitochondria and combined with oxaloacetate to form citrate (hence the other term of citric acid cycle or TCA cycle)). The cycle is shown below. Note that NADH and FADH2 are the high energy compounds that are produced in this cycle. The citric acid cycle and terminal oxidation are processes that occur within the mitochondria! Terminal oxidation - (oxidative phosphorylation) will generate ATP from the hydrogen ions and electrons that are taken from NADH and FADH2. For each NADH there will be three ATP generated and for each FADH2 there will two ATP generated. The NADH formed from glycolysis will only form 2 ATP due to the energy cost of moving these molecules into the mitochondria. Note where oxygen finally enters into the reaction chain. Energy Accounting: How many ATP’s are generated if glucose is taken completely through glycolysis, krebs cycle and oxidative phosphorylation? What is the net gain of ATP’s? How many ATP’s are generated if glucose is taken through glycolysis to pyruvate and then to alcohol? Protein Structure (conformation) - The chain of amino acids that are produced is termed the Primary structure of the protein. Nearby amino acids are brought into closer proximity to each other and they interact forming non-covalent hydrogen bonds forming Secondary structure. These secondary structures can form helices or sheets that bring more distant amino acids close together. Due to the folding by hydrogen bonds these more distant amino acids (if they are sulfur containing) can form disulfide “bridges” (bonds). This gives the amino acid chain further three dimensional shape called the Tertiary structure. If any of the three dimensional structures is changed, the activity of that enzyme will also be changed. Such a change is often referred to as denaturing of the protein. Plant Physiology: We can take all the chemicals that make up a cell, mix them together with water and we have a beaker full of diluted chemicals with no signs of cellular activity. One factor that distinguishes cells from a non-living system is compartmentalization of different cellular components (i.e. nucleus, chloroplasts, and other plastids) by cellular membranes. Cell membranes also have special proteins in their structure that help determine the movement (transportation) in and out of the cell of various substances. Exercise 3. - Effect of freezing on membrane proteins Anthocyanin is the coloring pigment in various plants, particularly beets. It is usually contained in the cell vacuole and kept inside by the vacuole membrane (tonoplast). Freezing can disrupt the vacoule membrane both with ice crystals and by causing membrane proteins to “fall out” of the membrane leaving holes. Slices of beets have been prepared that have either been refrigerated or frozen (and thawed). Take one slice of each treatment and place into separate test tubes, add water and observe. What is the difference between the two? How might you explain why the previously frozen beet slices show a difference from the unfrozen? Exercise 4. - Effect of freezing on enzyme compartmentalization in whole fruit Many enzymes are held in vesicles of other membrane bound “compartments” within the cell to avoid deleterious activity within the cell that is not desired. Freezing (and chilling in many tropical fruits) disrupts the membranes that kept these enzymes separated from the rest of the cell and allows them to leak into places they were not intended to be, leading to such effects as oxidative browning of the fruit peel. Examine the two bananas on display. One (A) has been placed in a refrigerator overnight while the other (B) was left at room temperature. What is the difference between the two? Explain what has happened to the refrigerated banana. We should all be aware that living systems have a variety of proteins that catalyze chemical reactions and allow for complex chemical reactions to occur, such as photosynthesis, respiration and metabolism. These protein catalysts are termed enzymes. One enzyme that is common to most living systems is catalase which can rapidly decompose hydrogen peroxide into water and oxygen. We will use pieces of potato that contain this enzyme. Exercise 5. - Effect of prolonged elevated temperature on enzymatic activity. Slices of potato have been either placed in water or heated to 60 OC for 15 minutes. Take a piece of each and place into separate test tubes. Fill test tubes with hydrogen peroxide to the top and plug with urethane foam leaving no air space. Invert the test tube and place upside down into a beaker of water and observe for accumulation of oxygen in the end of the test tube. What is the difference between the two? How might you explain the difference between the heated potato and the non-heated potato? Exercise 6. - Visible evidence of the effect of heavy metals and heat on proteins. Take egg white (contains mostly protein) in two test tubes. Heat the first test tube in a boiling water bath for 10 minutes, add a few drops of lead nitrate to the second test tube. (Caution! DO NOT ingest any of this material! Wash off any that you get on your hands) Compare the test tubes and explain the results. Exercise 7. - The effect of pH on enzymatic activity. All enzymes have a pH at which they work at maximum activity. This is termed the pH optimum for that enzyme. The pH optimum is different for various enzymes and is often contingent upon what environment the enzyme is “working” in. Using fresh unheated potato slices in hydrogen peroxide as for Exercise 5, place pieces of potato in separate test tubes of peroxide at pH 2, 7 and 14. Observe the rate of oxygen evolution over time. Which pH showed the greatest enzyme activity? Which pH showed the least enzyme activity? Which pH would you consider the optimum for the catalase enzyme in potato?