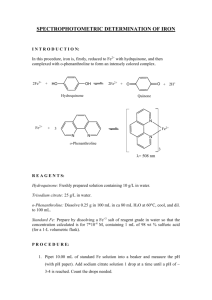
doi:10.1016/j.jmb.2005.05.071
J. Mol. Biol. (2005) 351, 799–809
The Periplasmic Binding Protein of a Tripartite
Tricarboxylate Transporter is Involved in
Signal Transduction
Rudy Antoine1, Isabelle Huvent2, Karim Chemlal1, Isabelle Deray1
Dominique Raze1, Camille Locht1 and Françoise Jacob-Dubuisson1*
1
INSERM U629, Institut de
Biologie de Lille, Institut
Pasteur de Lille, 1 rue Calmette
59019 Lille Cedex, France
2
CNRS UMR 8525, Institut de
Biologie de Lille, Institut
Pasteur de Lille, 1 rue Calmette
59019 Lille Cedex, France
A new type of solute importer has been identified recently in various
bacterial genera and called the tripartite tricarboxylate transporter (TTT).
TTTs consist of two cytoplasmic membrane proteins and a periplasmic
solute-binding protein. In the whooping cough agent Bordetella pertussis, a
TTT system that has been called BctCBA mediates the uptake of citrate,
with BctA and BctB being the membrane components and BctC, the
periplasmic protein. Here, we describe that the expression of the bctCBA
operon is induced by the presence of citrate in the milieu. The signalling
cascade involves both BctC and the signal transduction two-component
system BctDE, encoded by an operon adjacent to bctCBA. Furthermore,
two-hybrid analyses and affinity chromatography experiments indicated
that citrate-liganded BctC interacts with the periplasmic domain of the
sensor protein, BctE. Thus, BctC is part of the signalling cascade leading to
upregulation of the transporter operon in the presence of its solute, a new
function for periplasmic binding proteins of TT transporters.
q 2005 Elsevier Ltd. All rights reserved.
*Corresponding author
Keywords: tripartite tricarboxylate transporter (TTT); periplasmic binding
protein; two-component system; Bordetella pertussis; signal transduction
Introduction
Uptake and efflux of solutes are mediated by
transport systems embedded in the plasma membrane.1 In bacteria, several types of uptake transporters
have
incorporated
periplasmic
(‘extracytoplasmic’ in the case of Gram-positive
bacteria) solute-binding proteins that scavenge
their specific ligand(s) with high affinity and feed
them to their cognate membrane components.2 ABC
transporters, tripartite ATP-independent (TRAP)
transporters, and the more recently described
tripartite tricarboxylate transporters (TTT) all
depend on periplasmic solute-binding proteins.3–6
The prototypic TTT is TctCBA of Salmonella
typhimurium, with TctC being a periplasmic
citrate-binding protein and TctA and TctB two
integral membrane proteins predicted to contain
four and 12 transmembrane segments, respectively.7–9 tctCBA are encoded by a single operon.
Abbreviations used: TRAP, tripartite ATP-independent
transporter; TTT, tripartite tricarboxylate transporter.
E-mail address of the corresponding author:
francoise.jacob@ibl.fr
Recently, we have identified a TTT system in
Bordetella pertussis, the etiologic agent of whooping
cough, and shown that it is involved in citrate
uptake like its S. typhimurium orthologue.10 We thus
propose to name the three B. pertussis proteins BctC,
BctB and BctA, by analogy with their Salmonella
orthologues (Bct is for Bordetella citrate transporter).
In addition to S. typhimurium tctCBA and B. pertussis
bctCBA, several operons coding for homologous
systems have been identified in various bacterial
genera, but their function and regulation are
unknown.6,10
B. pertussis bctC was formerly called bug4 because
it is part of the large bug (for Bordetella uptake genes)
family, whose members have been identified in a
number of bacteria.10 Interestingly, the genome of
B. pertussis contains 78 bug paralogues, while, in
contrast, it carries only two operons coding for
membrane components of TTT systems, including
bctCBA. This raises intriguing questions about the
functions of all the putative Bug proteins.10 In
B. pertussis, BctC and several other Bug proteins are
produced at high levels, arguing for their importance in the lifestyle of the bacterium.10 In addition
to those of Bordetella, a few other bacterial genomes
0022-2836/$ - see front matter q 2005 Elsevier Ltd. All rights reserved.
800
show a tremendous expansion of the bctC/bug
gene family, whereas the bctBA homologues are
systematically much less abundant.10
TTT-encoding operons are often flanked by
operons encoding putative sensory transduction
two-component systems,6,10,11 although the regulation of their expression has not been studied. In
B. pertussis, bctCBA is preceded by an operon
encoding a two-component system that we have
named BctDE. In this work, we investigated the
regulation of TTT expression in B. pertussis.
The bctCBA operon is positively regulated by the
presence of citrate in the growth medium. Both the
BctDE two-component system and the periplasmic
protein BctC are necessary for this regulation. Thus,
the binding protein is both the periplasmic component of the citrate transporter and a component
of a citrate signalling machinery. This represents a
new function for periplasmic binding proteins of
TTT systems.
Results
Induction of the bctCBA operon by citrate
We have shown that BctCBA is involved in citrate
uptake by B. pertussis.10 To determine whether the
bctCBA operon is induced by the presence of citrate
in the growth medium, a promoterless lacZ
reporter gene was inserted into the chromosome
of B. pertussis BPSM by homologous recombination
immediately after the termination codon of bctA,
yielding BPSM/bctA-lacZ (Figure 1(a), first line).
A long predicted hairpin likely corresponding to
the terminator of the operon was not included in
this construct, in order to generate a transcriptional
fusion placing the reporter gene under the control
of the promoter of the bctCBA operon, while
keeping the operon fully functional. BPSM/bctAlacZ was grown to mid-exponential phase in liquid
SS medium under agitation, and the bacterial
suspension was then split into two and one half
supplemented with 10 mM citrate. The b-galactosidase (b-gal) activities were measured after two
hours of further incubation. Bacteria not treated
with citrate produced approximately 20 b-gal units,
whereas two hours of treatment with 10 mM citrate
increased their b-gal activity by approximately
15-fold (Table 1; Figure 1(b), panel 1). It is
noteworthy that the untreated controls showed
significant b-gal activities, suggesting a basal
transcription of the bctCBA operon without added
citrate. Alternatively, since the medium for the
B. pertussis cultures is not fully defined, it cannot
be excluded that it contains trace amounts of
citrate.
Involvement of the BctDE two-component
system in the regulation of the bctCBA operon
Located immediately upstream of bctCBA lies an
operon encoding a putative two-component
Periplasmic Binding Protein Involved in Signalling
system,10 with BctD being a predicted response
regulator and BctE a predicted sensory transducer
(Figure 1(a)). Schematically, two-component
systems are composed of a sensor-kinase in the
cytoplasmic membrane and a response regulator in
the cytoplasm, and they function as follows. The
detection of a periplasmic signal by the sensorkinase protein triggers a signalling cascade that
results in the phosphorylation of the cytoplasmic
response regulator, often acting as a transcriptional
regulator.12,13 Two-component systems in bacteria
frequently regulate the expression of genes adjacent
to their own. We therefore decided to test the
hypothesis that bctDE is involved in the regulation
of bctCBA.
Operons homologous to bctDE are found in the
vicinity of bctCBA-like operons in several other
bacteria, and in most cases the two sets of genes are
transcribed divergently.10 In contrast, bctDE is
transcribed in the same direction as bctCBA in
B. pertussis. However, the distance between the last
codon of the former and the first one of the latter,
more than 120 bp, makes it likely that bctDE and
bctCBA form separate transcriptional units. This
was confirmed by generating a lacZ fusion by
homologous recombination of a suicide plasmid at
the 3 0 end of bctDE in BPSM, yielding BPSM/bctDElacZ (Figure 1(a), line 3). Since disruptions of bctC or
bctA have been shown to abolish citrate uptake,10
we reasoned that a large genetic insertion
between bctE and bctC would have a negative
polar effect on downstream transporter genes if
bctDE and bctCBA form a single transcriptional unit.
The ability of the recombinant strain to import
citrate was thus measured as described.10 BPSM/
bctDE-lacZ was proficient in citrate uptake, similar
to its parent, BPSM, confirming that bctDE and
bctCBA are not part of a single operon (not shown).
However, we cannot totally exclude a contribution
of the promoter of bctDE to basal transcription of
bctCBA.
To investigate the potential involvement of BctDE
in the regulation of bctCBA, a large deletion of the
bctDE genes was then generated in the chromosome
of BPSM by allelic exchange, yielding BPSMDbctDE.
A transcriptional fusion was then created by
inserting lacZ at the 3 0 end of the bctCBA operon
in BPSMDbctDE to yield BPSMDbctDE/bctA-lacZ
(Figure 1(a), line 2). No b-gal activity was detected,
regardless of the presence of citrate in the growth
medium (Table 1; Figure 1(b)). Therefore, the twocomponent system BctDE is required both for
citrate induction of the bctCBA operon and for
basal transcription of bctCBA in the absence of
added citrate.
To investigate a potential autoregulation of
bctDE, the b-gal activity of BPSM/bctDE-lacZ was
determined. The recombinant strain exhibited
low levels of b-gal activity, regardless of the
presence of citrate in the milieu (Figure 1(b),
panel 3; Table 1). Thus, the bctDE operon is not
activated by citrate, and therefore it is not autoregulated.
Periplasmic Binding Protein Involved in Signalling
801
Figure 1. (a) A representation of the bct locus and positions of the lacZ transcriptional fusions. The deletions of bctDE
(line 2) and bctC (line 7) are represented by truncated arrows. (b) Levels of b-galactosidase activity corresponding to the
transcriptional fusions shown in (a). The C and K symbols indicate addition and no addition of 10 mM citrate to
the growth medium, respectively. Asterisks indicate that b-galactosidase activities were below detection levels. Note that
the scales may differ between panels.
802
Periplasmic Binding Protein Involved in Signalling
Table 1. b-Galactosidase activities of recombinant B. pertussis
Genetic background and transcriptional
fusion
BPSM/bctA-lacZ
BPSM DbctDE/bctA-lacZ
BPSM/bctDE-lacZ
BPSM/bctATlacZ
BPSM/bctC-lacZ
BPSM/bctCTlacZ
BPSM DbctC/bctA-lacZ
b-Galactosidase activity (arbitrary units) in
SS
SSCcitrate
15G2
!5
15G4
579G18
3379G57
80G1
27G2
253G17
!5
14G3
713G41
4058G219
84G1
28G6
The measurements were performed at least three times independently for each strain and for each condition.
Role of the components of the BctCBA transport
system in citrate-induced upregulation of
bctCBA
We next investigated the relationship between
citrate transport and regulation of the bctCBA
operon. The disruption of bctA in BPSM/bctATlacZ
has been shown to abolish citrate uptake by
B. pertussis.10 Taking advantage of the lacZ transcriptional fusion in BPSM/bctATlacZ (Figure 1(a),
line 4), we measured the b-gal activities of cultures
with or without added citrate. In the absence of
citrate, the levels of reporter activity in BPSM/
bctATlacZ were 38-fold higher than with the intact
BctCBA transporter (Table 1; Figure 1(b), compare
panels 1 and 4). These levels were increased only
slightly by the addition of citrate to the milieu
(Figure 1(b); Table 2).
When the lacZ fusion was inserted at the 3 0 end of
bctC (Figure 1(a), line 5), which abolishes citrate
transport by a polar effect on bctBA (not shown), the
b-gal activity of the resulting BPSM/bctC-lacZ was
approximately sixfold higher than that of BPSM/
bctATlacZ. Again the addition of citrate increased
the activity only slightly (Figure 1(b), panel 5;
Table 1).
It thus appears that the highest levels of bctCBA
expression are obtained when membrane components of the transport apparatus, BctA and/or
BctB are missing, which abolishes citrate uptake.
One explanation for this situation is that all the
citrate present in the milieu in the absence of
transport is available for signalling and thus for
induction of bctCBA expression. The observation
that the levels of activity of the two knocked-out
strains depended on the position of the reporter
gene in the operon indicates that bctC is transcribed
at a higher level than bctA.
Two other transport mutants were tested similarly. Interestingly, the disruption of bctC, and
consequently of the entire operon, in BPSM/
bctCTlacZ, resulted in b-gal activities significantly
Table 2. Oligonucleotides used in this study
bctDE-Up1
bctDE-Lo1
bctDE-Up2
bctDE-Lo2
bctC-Up1
bctC-Lo1
bctC-Up2
bctC-Lo2
bctDE-Fus-Up
bctDE-Fus-Lo
bctA-In-Up
bctA-In-Lo
bctCBA-Fus-Up
bctCBA-Fus-Lo
bctC-In-Up
bctC-In-Lo
bctC-Fus-Up
bctC-Fus-Lo
bctC-Hyb-Up
bctC-Hyb-Lo
bctE-Hyb-Up
bctE-Hyb-Lo
bug12-Hyb-Up
bug12-Hyb-Lo
BP3137-Hyb-Up
BP3137-Hyb-Lo
bctE-GB-Up
bctE-GB-Lo
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
50
GAATTCGCCGCGCCGCTGATTTA 3 0
AATGCGCATGGCTGGATTTTG 3 0
CAAAATCCAGCCATGCGCATTGCGGCACTGGTGTTTTCCCTA 3 0
TCTAGAGCCGGGGCAATGCACTCG 3 0
GAATTCCAGCGAAGTCACGGTCAAGGT 3 0
GAATTTCGCCAGGGTATGC 3 0
GCATACCCTGGCGAAATTCTTCGGCCTCATCAAGAAGTAA 3 0
TCTAGACACTGGAACAGGAACGCATAG 3 0
AAGCTTGGACCTGGGGCTGGATGT 3 0
GGTACCGCCTTTCCGGAACCTTTCAGA 3 0
AAGCTTGGTCGCGGCTATCGGTTCGTT 3 0
GGTACCCGCCAGCACGCCCTTGAC 3 0
AAGCTTATCGCCAACGTCCTGCTGTT 3 0
GGTACCCTACGACTGAGCGGCTTGCCT 3 0
GGTACCGGTCTTGCTGAACGGGAACA 3 0
AAGCTTATCCGCAACGATTCGCCCTAT 3 0
AAGCTTCGAAGGTGGCGGTGAG 3 0
GGTACCCGGTTTACTTCTTGATGAGGC 3 0
AGATCTCGATGAACCGCGTCGGCCCGAG 3 0
GGTACCCGGTTTACTTCTTGATGAGGC 3 0
GGTACCGTCCAACCAGCAACTGCGCAA 3 0
AAGCTTCGTTCCACCGAGCGCACCAGC 3 0
TAGGATCCGCGCATCGTCGTCCC 3 0
ATGGTACCTGCTTGTTCCTCGTTATTCG 3 0
ATGGTACCGTACCTGACGTTCGACAAGA 3 0
ATAAGCTTTCGTTGTCGGTGGCGTC 3 0
AGATCTTCCAACCAGCAACTGCGCAAC 3 0
CTCGAGACGTTCCACCGAGCGCACCA 3 0
Periplasmic Binding Protein Involved in Signalling
lower than those observed for BctC-producing
BPSM/bctATlacZ and BPSM/bctC-lacZ (Figure 1,
compare panel 6 to panels 4 and 5; Table 1). The
levels of activity were independent of citrate
addition to the milieu. Similarly, when a non-polar
bctC mutant was tested containing a lacZ transcriptional fusion at the 3 0 end of bctCBA (BPSMDbctC/
bctA-lacZ, Figure 1(a), line 7), the b-gal activities
were even lower than those of BPSM/bctCTlacZ
and, again, independent of the addition of citrate
(Figure 1(b), panel 7; Table 1). Thus, in the absence
of BctC, the expression of bctCBA is low, in sharp
contrast with the strains lacking only the membrane
proteins of the transporter, and it is uninducible by
citrate.
In addition to the two-component system BctDE,
BctC appears to be required for the induction of the
bctCBA operon by citrate (Figure 1(b), compare
panels 1 and 7). However, in the absence of BctC,
BctDE is sufficient for low-level transcription of
bctCBA (Figure 1(b), panel 7).
Interaction of BctC with the periplasmic domain
of BctE
Since both BctDE and BctC are required for citrate
induction of the bctCBA operon, it is possible that
803
citrate signalling between the periplasm and the
cytoplasm involves interactions between the sensor
protein BctE and the periplasmic receptor. BctE is
predicted to have two transmembrane segments
separated by a periplasmic domain and followed by
a C-terminal kinase domain in the cytoplasm (not
shown). To investigate a potential interaction
between BctC and the predicted periplasmic
domain of BctE (Ser21-Arg164), an Escherichia coli
two-hybrid system was used.14 BctC and the BctE
domain were produced in E. coli as separate
chimeric proteins with the complementary domains
T25 and T18 of adenylate cyclase, respectively.
Induction of the maltose utilization operon by the
cAMP–CRP complex requires active adenylate
cyclase, which can be obtained by bringing
together T18 and T25 thanks to the interactions of
the two proteins fused to these adenylate
cyclase domains. The introduction of both
pT25BctC-Hyb and pT18BctE-Hyb into cyadeficient E. coli BTH101 resulted in red colonies on
McConkey agar containing 1% maltose, while
bacteria containing only one or none of the hybrids
remained white (Figure 2(a)). This suggests
that BctC interacts with the periplasmic domain of
BctE.
To assess the specificity of this interaction, we
Figure 2. Two-hybrid analyses in E. coli. (a) bctC and bctE were inserted into pT25 and pT18, yielding pT25BctC-hyb
pT18BctE-hyb, respectively. These two plasmids were introduced together into E. coli BTH101, and the recombinant
clones were plated onto MacConkey agar containing 1% (w/v) maltose. As negative controls, pT25BctC-hyb or
pT18BctE-hyb were introduced into the same host bacteria with pT18 or pT25, respectively. The clones that were able to
utilize maltose grew as red colonies, indicating a positive two-hybrid response. (b) B. pertussis bug12 and the sequence
coding for the periplasmic domain of the putative B. pertussis sensor protein BP3137 were inserted into pT25 and pT18,
yielding pT25bug12-Hyb and pT18BP3137-Hyb, respectively. Various plasmid combinations as indicated in the Figure
were introduced into E. coli BTH101, and the recombinant clones were plated onto maltose McConkey agar.
804
chose another B. pertussis Bug protein, Bug12
(BP0334), and produced it as a chimeric protein
with the T25 domain of adenylate cyclase. Similarly,
we chose another sensor protein of B. pertussis
whose predicted topology is similar to that of
BctE, BP3137, and produced its periplasmic domain
as a chimeric protein with the T18 domain of
adenylate cyclase. The resulting pT25Bug12-Hyb
and pT18BP3137-Hyb plasmids were then used in
two-hybrid experiments in combination with
pT18BctE-Hyb and pT25BctC-Hyb, respectively
(Figure 2(b)). Neither BTH101(pT25Bug12-Hyb,
pT18BctE-Hyb) nor BTH101(pT25BctC-Hyb,
pT18BP3137-Hyb) yielded red colonies on
maltose McConkey agar, in contrast with
BTH101(pT25BctC-Hyb, pT18BctE-Hyb). Similarly,
BTH101(pT25Bug12-Hyb, pT18BP3137-Hyb) bacteria were unable to utilize maltose. These data
suggest strongly that the interaction between
BctC and the periplasmic domain of BctE is
specific.
In addition to the two-hybrid method, an in vitro
approach was used to confirm the interaction
between BctC and the periplasmic domain of
BctE. We reasoned that by immobilizing one of the
two partners on a chromatography column it
should be possible to detect the binding of the
second protein to the first one, and we thus devised
an affinity chromatography method. The periplasmic domain of BctE was produced as part of a
chimeric GB1-BctEp-His6 protein. GB1 is a small
protein domain of 56 residues with a strong affinity
for human IgG.15 The hexa-histidine tag was used
to purify the recombinant GB1-BctEp-His6 protein,
which was then bound to IgG-Sepharose thanks to
the IgG-binding properties of GB1. The second
protein, BctC, was applied onto thus immobilized
BctE in the form of a crude lysate of BPSM/bctClacZ, in which bctC is transcribed at a high level
independent of the addition of citrate (see above).
The lysate was prepared in the presence of citrate.
Because of the possibility that several proteins of
the crude lysate bind the Sepharose matrix or the
recombinant protein in a non-specific manner, a
BctC-less lysate was prepared in a similar fashion
using BPSMDbctC. The GB1-BctEp-His6 protein was
then eluted from the IgG column, together with any
proteins bound to it. Only one protein whose
molecular mass was compatible with that calculated for BctC differed between the two samples as
detected by staining SDS/polyacrylamide gels with
Coomassie brilliant blue (Figure 3(a)). The protein
was analysed by peptide mass fingerprinting, and
eight matching peptides mapping throughout the
protein identified it unequivocally as BctC (32%
peptide coverage). A control experiment showed
that BctC does not bind to the IgG-Sepharose beads,
independent of the presence of GB1-BctE (not
shown).
This distinct experimental approach argues
strongly in favour of specific interactions between
BctC and the periplasmic domain of BctE, corroborating the two-hybrid results.
Periplasmic Binding Protein Involved in Signalling
Figure 3. BctC–BctE interaction as evidenced by affinity
chromatography. (a) A recombinant GB1-BctEp-His6
protein containing the predicted periplasmic domain of
BctE was purified by metal chelate chromatography and
then immobilized on IgG-Sepharose beads. Crude lysates
of BPSM DbctC (lane 1) or BPSM/bctC-lacZ (lane 2)
prepared with citrate were applied onto two aliquots of
IgG-Sepharose beads thus conditioned. After washing
with buffer, GB1-BctEp-His6 was eluted from the IgGSepharose beads together with the proteins bound to it by
a pulse of acetic acid, and the two eluates were analysed
by SDS-PAGE. The gels were stained with Coomassie
brilliant blue to visualise proteins that co-eluted with
GB1-BctEp-His6. One protein was conspicuously present
in the sample obtained with the BPSM/bctC-lacZ lysate
and absent from the other sample. Mass fingerprinting
analyses identified it unambiguously as BctC, with eight
matching tryptic peptides (see Supplementary Data). (b)
BPSM/bctC-lacZ lysates were prepared from liquid
cultures grown in SS medium (lane 1) or in SS medium
supplemented with 10 mM citrate (lane 2). The affinity
chromatography experiments were performed as above,
in buffers without citrate (lane 1) or supplemented with
10 mM citrate (lane 2). The eluates were analysed as
above. BctC was identified in the second eluate by mass
fingerprinting analysis.
Citrate-loaded BctC, but not the unliganded
protein, interacts with BctE
For the transduction system to be inducible, only
citrate-liganded BctC should interact with the
periplasmic domain of BctE, and not free BctC. An
alternative, less likely, possibility is that unliganded
BctC interacts with BctE in a non-productive
manner, and citrate binding triggers a conformational change in BctC to activate the signalling
cascade. To distinguish between these possibilities,
we attempted to detect a BctC–BctE interaction in
the absence of citrate.
The detection of the interaction by the two-hybrid
system implies that the two proteins are produced
in the cytoplasm of E. coli. Though no citrate was
added to the plates in these experiments, it is quite
plausible that BctC traps some of the intrinsic
cytoplasmic citrate. This is suggested strongly by
the fact that several attempts to overproduce
cytoplasmic BctC in E. coli proved unsuccessful
(not shown), arguing that the protein is toxic under
those conditions, probably because it titrates the
intracytoplasmic citrate. We therefore chose to
Periplasmic Binding Protein Involved in Signalling
compare the binding of unliganded versus liganded
BctC to BctE by affinity chromatography. It should
be recalled that in those conditions, BctC is
produced in the periplasm, rather than the cytoplasm, of B. pertussis. One half of the sample was
prepared from BPSM/bctC-lacZ grown in the
absence of citrate, and no citrate was added at any
step of the experiment. We reasoned that under
those conditions, the majority of BctC molecules
should not be liganded. The other half was
prepared from citrate-treated cultures, and 10 mM
citrate was added to the buffers used to prepare
the lysate and to perform the chromatography
affinity experiment. As shown in Figure 3(b), no
BctC was bound to BctE using the sample prepared
without citrate, in contrast to the sample prepared
in the presence of citrate, in which BctC was
identified by mass fingerprinting analysis as
above (not shown). These data indicate that BctC
has to be citrate-loaded in order to interact with
BctE.
Discussion
The TTT system BctCBA mediates citrate import
in B. pertussis.10 In this work, we have shown that
the levels of transcription of the bctCBA operon
depend on the presence of citrate in the growth
medium, probably to maximize citrate uptake
when it becomes available in the milieu. In the
absence of citrate, the transport system is produced
in small amounts. The two-component system
BctDE is required for this transcriptional
regulation, as shown by the lack of detectable
bctCBA transcription or induction in bctDEdeficient B. pertussis. The regulation of genes
coding for solute transporters by two-component
systems encoded by adjacent operons is rather
common in bacteria.5,11,16–18 However, BctDE is not
sufficient for citrate induction of bctCBA
expression, since the periplasmic component of
the transporter, BctC, is required. BctDE is necessary and sufficient for basal transcription of bctCBA
in the absence of citrate, while both BctC and BctDE
are required for citrate signalling leading to
upregulation.
The following lines of evidence sustain the
role of BctC in signal transduction. When citrate
uptake is abolished by the disruption of the
bctCBA operon, the level of transcription of
bctCBA depends on the presence of BctC. In
the absence of the periplasmic binding protein,
bctCBA is transcribed at low levels and is not
inducible by citrate. In contrast, when BctC is
produced but the membrane components of the
transporter are lacking, very high levels of
bctCBA expression are obtained, even without
the addition of citrate. Furthermore, liganded
BctC interacts directly with the periplasmic
domain of the sensory transducer BctE, as
evidenced by two-hybrid experiments and affinity chromatography. Therefore, we propose that
805
an interaction between citrate-loaded BctC and
the periplasmic domain of BctE activates the
signal transduction cascade, resulting in upregulation of the transporter operon (Figure 4).
When all the components of the transporter and
signalling systems are present, citrate-loaded
BctC most likely partitions between the transport
and the signalling pathways by interacting with
BctBA and BctE, respectively. This model provides an explanation for the high levels of
bctCBA transcription when BctA and BctB are
lacking, as all the liganded BctC is then
exclusively available for signalling.
At least two lines of evidence indicate that the
Bct signalling system is highly sensitive to
citrate. Firstly, even the addition of as little as
1 mM citrate to the bacterial cultures was
sufficient to fully induce the transcription of
bctCBA, suggesting that such a concentration is
saturating even when citrate is imported at the
same time (our unpublished results). Secondly,
in the absence of BctC, very high levels of
bctCBA expression were obtained without adding citrate to the milieu, and they were raised
only slightly by the addition of citrate. This
suggests that the non-supplemented B. pertussis
growth medium used in this study contains
Figure 4. Model of the regulation of the TTT operon in
B. pertussis. Under uninduced conditions, small amounts
of the BctCBA transporter are produced. When citrate is
available in the milieu, it binds to BctC. Citrate-loaded
BctC then partitions between the transport pathway,
through interactions with BctBA, and the signalling
pathway, through interactions with the periplasmic
domain of the sensor-kinase BctE. Activation of the signal
transduction cascade by the BctC/BctE interaction results
in the upregulation of bctCAB transcription, and hence in
the production of additional transporter molecules. BctC
is represented as a bilobate protein with the citratebinding site located between the two domains. BctA and
B are predicted to have 12 and 4 transmembrane
segments, respectively. BctE is predicted to have a
periplasmic domain, a cytoplasmic domain and two
transmembrane segments. BctD is the cytoplasmic
response regulator of the BctDE two-component system.
OM and IM denote the outer and inner membranes,
respectively. P denotes a promoter. Genes of the bct locus
are represented as arrows.
806
trace amounts of citrate sufficient to upregulate
bctCBA transcription, provided the BctC–BctDE
signalling cascade is complete and the membrane components of the transport system are
lacking. Under these conditions, the level of
bctCBA transcription is not raised much further
by the addition of citrate to the milieu. In
contrast, these trace amounts appear not to be
sufficient to activate the signalling cascade when
the transporter is functional, most likely because
they are imported quickly.
The bctDE operon is transcribed at a low level,
and it is not citrate-inducible. Its constitutive
expression probably ensures low-level transcription
of bctCBA under non-inducing conditions. The
small amounts of BctC thus constitutively produced
most likely ensure that signal transduction can be
triggered as soon as the inducer is detected in the
environment.
Although the involvement of a periplasmic
solute-binding protein of the Bug/TctC family in
signal transduction is a new feature of TTT systems,
extracytoplasmic solute-binding proteins of other
bacterial transport systems participate in signal
transduction.2,19 For instance, in addition to its
involvement in maltose uptake as the periplasmic
component of an ABC transporter, MalE has been
shown to interact with the Tar methyl-acceptor
chemotaxis protein (MCP).20,21 Probably the most
striking example of a multi-functional periplasmic
solute-binding protein is Agrobacterium tumefasciens
ChvE, involved in the uptake of monosaccharides
released by the wounded plant upon infection, in
chemotaxis towards these compounds and in
signalling their presence via its interaction with
the sensor kinase VirA.22–25 Conversely, the
succinate-binding protein YdbE of Bacillus subtilis
is an extracytoplasmic protein dedicated to signal
transduction only. Together with a two-component
system, it is required for the transcriptional
activation of the gene coding for the C4-dicarboxylic acids transporter YdbH, while it appears
not to participate in succinate uptake by YdbH.26
BctC is the first Bug protein of B. pertussis with a
known function. According to phylogenetic analyses of the family, it is the closest homologue of
S. typhimurium TctC and other orthologues that are
part of TTT systems.6,10 All other Bug proteins of
B. pertussis diverge widely from the canonical
citrate-binding proteins, and no other bug gene is
part of a TTT-encoding operon. This diversity
precludes the easy prediction of their functions.
We have recently solved the X-ray structures of two
Bug proteins of B. pertussis with their ligands, which
are different from citrate in both cases (I.H.,
unpublished results). While it is likely that these
proteins participate in solute uptake, the membrane
transporters with which they cooperate remain to
be identified. Alternatively, or in addition, other
Bug proteins may participate in signal transduction
cascades to activate transport or metabolic pathways in response to the presence of their specific
ligands.
Periplasmic Binding Protein Involved in Signalling
Materials and Methods
Construction of the B. pertussis recombinant strains
The B. pertussis Tohama I derivative BPSM has been
described.27 The sequences of all the oligonucleotides
used as PCR primers are shown in Table 2. To generate a
deletion of bctC in BPSM, DNA fragments flanking the
gene were amplified by PCR using the pairs of
oligonucleotides bctC-Up1 and bctC-Lo1, and bctC-Up2
and bctC-Lo2, respectively. These oligonucleotides were
designed to generate a short overlap between the two
amplicons, which were thus annealed to serve as the
matrix for a third PCR using bctC-Up1 and bctC-Lo2 as
primers. The final amplicon was inserted into
pJQ200mp18rpsl (see below), and the recombinant
plasmid was used to generate the bctC deletion by allelic
exchange as described,28 yielding BPSMDbctC.
To construct pJQ200mp18rpsl, the rpsl allele was
amplified by PCR using pSS1129 as a template,28 and
the oligonucleotides 5 0 GTAACCGCTACCTTGAAAGTC
3 0 and 5 0 ATCGATGGCAGAATTTTACGCTGAC 3 0 as
primers. The 816 bp amplicon was restricted with ClaI
(underlined) and the fragment inserted into ClaI/HpaIrestricted pJQ200mp1829 in replacement of the sacB gene,
thus yielding pJQ200mp18rpsl.
To generate a deletion of bctDE in BPSM, DNA
fragments flanking the bctDE operon were amplified by
PCR using the two pairs of oligonucleotides; bctDE-Up1
and bctDE-Lo1, and bctDE-Up2 and bctDE-Lo2, respectively. The procedures for the design of the oligonucleotides, the PCR amplification and the allelic exchange were
similar to those described above for the bctC deletion. The
resulting strain was called BPSMDbctDE. The two
deletions were verified by PCR.
All the other recombinant strains were obtained by the
procedure outlined below, using the suicide vector pFUS2
as described.30 Briefly, following the insertion of the
relevant PCR amplicons into pFUS2 (see below), the
recombinant plasmids were introduced into the recipient
B. pertussis strain by conjugation using E. coli SM10 as the
donor strain. The suicide vectors were inserted into the
chromosome by homologous recombination, generating
transcriptional fusions between each target site and
pFUS2-encoded lacZ. In addition, the transcriptional
terminator of pFUS2 resulted in the disruption of the
operon following the insertion site in the chromosome.30
To create a lacZ transcriptional fusion at the 3 0 end of
the bctDE operon, the 3 0 portion of bctE was amplified
using the oligonucleotides bctDE-Fus-Up and bctDE-FusLo as primers. The recombinant strain BPSM/bctDE-lacZ
was generated as outlined above. To create a lacZ
transcriptional fusion within bctA, an internal fragment
of bctA was amplified by PCR using the oligonucleotides
bctA-In-Up and bctA-In-Lo as primers, yielding the
recombinant strain BPSM/bctATlacZ. To create a lacZ
transcriptional fusion at the 3 0 end of the bctCBA operon,
the 3 0 portion of bctA was amplified by PCR using the
oligonucleotides bctA-Fus-Up and bctA-Fus-Lo as
primers, yielding recombinant BPSM/bctA-lacZ. The
same pFUS2 derivative was used to generate
BPSMDbctDE/bctA-lacZ and BPSMDbctC/bctA-lacZ.
To both disrupt bctC and create a lacZ transcriptional
fusion within the first gene of the operon, an internal
fragment of bctC was amplified by PCR using the bctC-InUp and bctC-In-Lo oligonucleotides as primers, yielding
recombinant BPSM/bctCTlacZ. To create a lacZ transcriptional fusion at the 3 0 end of bctC, without disrupting the
807
Periplasmic Binding Protein Involved in Signalling
gene, the 3 0 portion of bctC was amplified by PCR using
the bctC-Fus-Up and bctC-Fus-Lo oligonucleotides as
primers, yielding recombinant BPSM/bctC-lacZ.
All PCR fragments were first inserted into pCRII-Topo
(InVitrogen) and sequenced using a Perkin Elmer 377
automatic sequencer.
Culture conditions
B. pertussis was grown on Bordet Gengou (BG) agar
with 10% (v/v) sheep blood as a solid medium or in
liquid modified Stainer Scholte (SS) medium under
agitation as described.31 The media were supplemented
with the appropriate antibiotics (100 mg/ml of streptomycin, plus 10 mg/ml of gentamycin for the strains
carrying pFUS2 derivatives).
Measurement of b-galactosidase activities
The recombinant B. pertussis strains were streaked onto
BG agar plates and incubated for two days at 37 8C, and
the bacteria were then scraped from these plates and used
to inoculate small volumes of SS medium. After 24 h of
incubation at 37 8C under constant shaking, the smallscale cultures were diluted to an absorbance at 600 nm
(A600) of 0.05 and then grown until mid-exponential phase
(A600 1.5–2). Each culture was then split into two, and one
half was treated with 10 mM trisodium citrate (pH 7)
while the other was left untreated. After two hours of
incubation at 37 8C, the cells were harvested by centrifugation and resuspended in phosphate-buffered saline.
The cell densities were estimated by measuring the A600 of
the cell suspensions. The bacteria were broken by a
passage in a French pressure cell, and b-galactosidase
activities were measured as described.30
Two-hybrid analyses
The DNA sequences coding for mature BctC and for the
predicted periplasmic domain of BctE were amplified
by PCR using the pairs of oligonucleotides bctC-HybUp/bctC-Hyb-Lo and BctE-Hyb-Up/bctE-Hyb-Lo,
respectively. After insertion of the two amplicons into
pCRII-TOPO and sequencing, the BglII-KpnI fragment
corresponding to the bctC sequence was inserted into
pT25 (Hybrigenics, Paris, France), and the KpnI-HindIII
fragment corresponding to the bctE sequence was
inserted into pT18 (Hybrigenics, Paris, France), yielding
pT25BctC-Hyb and pT18BctE-Hyb, respectively. These
two plasmids were introduced together into cya-deficient
E. coli BTH101, and the recombinant clones were plated
onto MacConkey agar containing 1% (w/v) maltose as
described.14 As negative controls, pT25BctC-Hyb or
pT18BctE-Hyb were introduced into the same host
bacteria with pT18 or pT25, respectively. The clones that
were able to utilize maltose grew as red colonies,
indicating a positive two-hybrid response.
Similar procedures were used to prepare the
pT25Bug12-hyb and pT18BP3137-hyb recombinant plasmids. The pairs of oligonucleotides bug12-Hyb-Up and
bug12-Hyb-Lo, and BP3137-Hyb-Up and BP3137-Hyb-Lo
were used as PCR primers to amplify bug12 and BP3137
by PCR, respectively. The amplicons were inserted into
pT25 and pT18. Various combinations of the four
recombinant plasmids were then used in two-hybrid
experiments as described above.
Analysis of BctC–BctE interactions by affinity
chromatography
The DNA sequence coding for the predicted periplasmic domain of BctE was amplified by PCR using the
oligonucleotides bctE-GB-Up and bctE-GB-Lo as primers.
After insertion into PCRII-Topo, sequencing and restriction by BglII and XhoI of the recombinant plasmid, the
BctE-encoding DNA fragment was inserted into BamHI/
XhoI-restricted pGEV2.15 The resulting plasmid, called
pGB-bctEp, was introduced into E. coli BL21(DE3)
(Novagen) by electroporation. The recombinant strain
was grown in liquid LB medium under agitation until
A600Z1, and the expression of the chimeric gene carried
by pGB-bctEp was induced by the addition of IPTG to
1 mM. After two more hours of culture, the bacteria were
collected by centrifugation, the pellet was resuspended in
0.1 volume of TS buffer (50 mM Tris–HCl (pH 7.5),
150 mM NaCl), and the bacteria were broken by two
passages in a French pressure cell. The clarified lysate was
applied onto a metal-chelate column (Ni-NTA agarose,
Qiagen) and the recombinant GB1-BctE-His6 protein was
eluted in 400 mM imidazole (pH 7). The eluate was
diluted fivefold in TS buffer and applied onto IgGSepharose beads (Pharmacia) conditioned as
recommended by the manufacturer. After ten minutes
rocking at room temperature to allow binding of the GB1
portion of the recombinant protein to IgG-Sepharose, the
beads were washed in TS buffer and then split into two
aliquots. BPSMDbctC and BPSM/bctC-lacZ were grown in
SS medium containing 10 mM trisodium citrate (pH 7),
unless stated otherwise, until the cultures reached midexponential phase, then the bacteria were harvested by
centrifugation, resuspended in TS buffer and broken by
passages in a French pressure cell. The clarified lysates
were then each applied onto an aliquot of IgG-Sepharose
beads prepared as described above. After rocking the
beads for ten minutes at room temperature with the
lysates to allow for the binding of protein(s) of the lysate
to the immobilized GB1-BctE-His6 protein, the beads
were harvested by low-speed centrifugation, washed
once in TS buffer and then treated with two volumes of
0.5 M acetic acid (pH 3.4) as recommended by the
manufacturer to disrupt the interaction between IgG
and GB1. Where indicated, the buffers were supplemented with 10 mM trisodium citrate (pH 7). After a
brief centrifugation to pellet the IgG beads, the GB1BctEp-His6 recombinant protein was thus recovered in
the eluates together with proteins bound to it. The eluates
were analysed by SDS-PAGE, and the proteins in the gels
were stained with Coomassie brilliant blue. A protein
found only in the sample obtained with the B. pertussis
lysate containing BctC was identified by mass fingerprinting analyses as described.32 In control experiments, the
clarified lysates of BPSMDbctC and BPSM/bctC-lacZ were
applied directly onto IgG-Sepharose beads conditioned in
TS buffer. The beads were rocked for ten minutes at room
temperature as described above, harvested by centrifugation and washed in TS buffer. Bound proteins were
recovered by a pulse of 0.5 M acetic acid (pH 3.4), and the
eluates were analysed by SDS-PAGE.
Acknowledgements
We thank Philip Supply for his insightful
808
comments on the manuscript and Hervé Drobecq
for mass spectrometry analyses. I.H. was supported
by the Région Nord-Pas-de Calais and K.C. by the
Programme ‘Gen-Homme’ of the Ministère de la
Recherche et de l’Industrie. F.J.-D. is a researcher of
the CNRS. This work was supported in part by
INSERM, the Institut Pasteur de Lille and the
Région Nord-Pas-de-Calais.
Supplementary Data
Supplementary data associated with this
article can be found, in the online version, at
doi:10.1016/j.jmb.2005.05.071
Periplasmic Binding Protein Involved in Signalling
15.
16.
17.
18.
References
1. Lolkema, J. S., Poolman, B. & Konings, W. N. (1998).
Bacterial solute uptake and efflux systems. Curr. Opin.
Microbiol. 1, 248–253.
2. Tam, R. & Saier, M. H., Jr (1993). Structural, functional,
and evolutionary relationships among extracellular
solute-binding receptors of bacteria. Microbiol. Rev. 57,
320–346.
3. Nikaido, H. & Hall, J. A. (1998). Overview of bacterial
ABC transporters. Methods Enzymol. 292, 3–20.
4. Rabus, R., Jack, D. L., Kelly, D. J. & Saier, M. H., Jr
(2001). TRAP transporters: an ancient family of
extracytoplasmic solute-receptor-dependent secondary active transporters. Microbiology, 145, 3431–3445.
5. Kelly, D. J. & Thomas, G. H. (2001). The tripartite ATPindependent periplasmic (TRAP) transporters of
bacteria and archae. FEMS Microbiol. Rev. 25, 405–424.
6. Winnen, B., Hvorup, R. N. & Saier, M. H., Jr (2003).
The tripartite tricarboxylate transporter (TTT) family.
Res. Microbiol. 154, 457–465.
7. Sweet, G. D., Kay, C. M. & Kay, W. W. (1984).
Tricarboxylate-binding
proteins
of
Salmonella
typhimurium. Purification, crystallization, and physical properties. J. Biol. Chem. 259, 1586–1592.
8. Widenhorn, K. A., Somers, J. M. & Kay, W. W. (1988).
Expression of the divergent tricarboxylate transport
operon (tctI) of Salmonella typhimurium. J. Bacteriol.
170, 3223–3227.
9. Widenhorn, K. A., Boos, W., Somers, J. M. & Kay,
W. W. (1988). Cloning and properties of the Salmonella
typhimurium tricarboxylate transport operon in
Escherichia coli. J. Bacteriol. 170, 883–888.
10. Antoine, R., Jacob-Dubuisson, F., Drobecq, H.,
Willery, E., Lesjean, S. & Locht, C. (2003). Overrepresentation of a gene family encoding extracytoplasmic solute receptors in Bordetella. J. Bacteriol. 185,
1470–1474.
11. Widenhorn, K. A., Somers, J. M. & Kay, W. W. (1989).
Genetic regulation of the tricarboxylate transport
operon (tctI) of Salmonella typhimurium. J. Bacteriol.
171, 4436–4441.
12. Hoch, J. A. (2000). Two-component and phosphorelay
signal transduction. Curr. Opin. Microbiol. 2, 165–170.
13. Stock, A. M., Robinson, V. L. & Goudreau, P. N. (2000).
Two-component signal transduction. Annu. Rev.
Biochem. 69, 183–215.
14. Karimova, G., Pidoux, J., Ullman, A. & Ladant, D.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
(1998). A bacterial two-hybrid system based on a
reconstituted signal transduction pathway. Proc. Natl
Acad. Sci. USA, 95, 5752–5756.
Huth, J. R., Bewley, C. A., Jackson, B. M., Hinnebusch,
A. G., Clore, G. M. & Gronenborn, A. M. (1997).
Design of an expression system for detecting folded
protein domains and mapping macromolecular interactions by NMR. Protein Sci. 6, 2359–2364.
Janausch, I. G., Zientz, E., Tran, Q. H., Kroger, A. &
Unden, G. (2002). C4-dicarboxylate carriers and
sensors in bacteria. Biochim. Biophys. Acta, 1553, 39–56.
Golby, P., Davies, S., Kelly, D. J., Guest, J. R. &
Andrews, S. C. (1999). Identification and characterization of a two-component sensor-kinase and
response-regulator system (DcuS-DcuR) controlling
gene expression in response to C4-dicarboxylates in
Escherichia coli. J. Bacteriol. 181, 1238–1248.
Hamblin, M. J., Shaw, J. G. & Kelly, D. J. (1993).
Sequence analysis and interposon mutagenesis of a
sensor-kinase (DctS) and response-regulator (DctR)
controlling synthesis of the high-affinity C4dicarboxylate transport system in Rhodobacter
capsulatus. Mol. Gen. Genet. 237, 215–224.
Kondoh, H., Ball, C. B. & Adler, J. (1979). Identification of a methyl-accepting chemotaxis protein for
the ribose and galactose chemoreceptors of Escherichia
coli. Proc. Natl Acad. Sci. USA, 76, 260–264.
Manson, M. D. & Kossmann, M. (1986). Mutations in
tar suppress defects in maltose chemotaxis caused by
specific malE mutations. J. Bacteriol. 165, 34–40.
Kossmann, M., Wolff, C. & Manson, M. D. (1988).
Maltose chemoreceptor of Escherichia coli: interaction
of maltose-binding protein and the tar signal
transducer. J. Bacteriol. 170, 4516–4521.
Winans, S. C. (1991). An Agrobacterium two-component regulatory system for the detection of
chemicals released from plant wounds. Mol. Microbiol.
5, 2345–2350.
Cangelosi, G. A., Ankenbauer, R. G. & Nester, E. W.
(1990). Sugars induce the Agrobacterium virulence
genes through a periplasmic binding protein and a
transmembrane signal protein. Proc. Natl Acad. Sci.
USA, 87, 6708–6712.
Shimoda, N., Toyoda-Yamamoto, A., Aoki, S. &
Machida, Y. (1993). Genetic evidence for an interaction between the VirA sensor protein and the ChvE
sugar-binding protein of Agrobacterium. J. Biol. Chem.
268, 26552–26558.
Kemner, J. M., Liang, X. & Nester, E. W. (1997). The
Agrobacterium tumefaciens virulence gene chvE is part
of a putative ABC-type sugar transport operon.
J. Bacteriol. 179, 2452–2458.
Asai, K., Baik, S. H., Kasahara, Y., Moriya, S. &
Ogasawara, N. (2000). Regulation of the transport
system for C4-dicarboxylic acids in Bacillus subtilis.
Microbiology, 146, 263–271.
Menozzi, F. D., Mutombo, R., Renauld, G., Gantiez, C.,
Hannah, J. H., Leininger, E. et al. (1994). Heparininhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect. Immun.
62, 769–778.
Stibitz, S. (1994). Use of conditionally counterselectable suicide vectors for allelic exchange. Methods
Enzymol. 235, 458–465.
Quandt, J. & Hynes, M. F. (1993). Versatile suicide
vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene, 127, 15–21.
Antoine, R., Alonso, S., Raze, D., Coutte, L., Lesjean,
S., Willery, E. et al. (2000). New virulence-activated
Periplasmic Binding Protein Involved in Signalling
and virulence-repressed genes identified by
systematic gene inactivation and generation of
transcriptional fusions in Bordetella pertussis.
J. Bacteriol. 182, 5902–5905.
31. Locht, C., Geoffroy, M.-C. & Renauld, G. (1992).
Common accessory genes for the Bordetella pertussis
filamentous hemagglutinin and fimbriae share
809
sequence similarities with the papC and papD gene
families. EMBO J. 11, 3175–3183.
32. Coutte, L., Antoine, R., Drobecq, H., Locht, C. &
Jacob-Dubuisson, F. (2001). Subtilisin-like autotransporter serves as maturation protease in a
bacterial secretion pathway. EMBO J. 20, 5040–5048.
Edited by I. B. Holland
(Received 11 February 2005; received in revised form 19 May 2005; accepted 31 May 2005)
Available online 23 June 2005