Osmosis and Diffusion Lab: AP Biology Experiments
advertisement

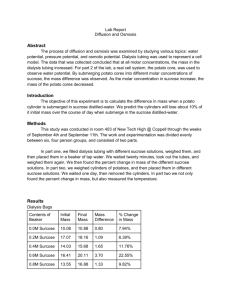
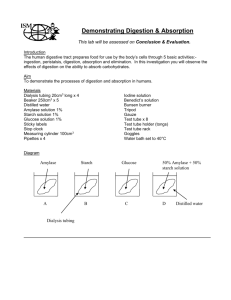
AP LAB 4 OSMOSIS AND DIFFUSION Overview: In this lab, you will conduct an investigation to demonstrate the processes of diffusion and osmosis and explain how diffusion is affected by various factors. Also to demonstrate and measure water potential, and explain how water potential is affected by pressure. Also to understand the effect of osmosis on living cells and calculate the water potential of living cells. Part 1: Diffusion in Agar Cells In this part of the lab, we will explore the relationship between diffusion and cell size. Procedure: 1. Calculate the surface area of each of the three agar cubes. Record in your data table. 2. Calculate the volume of each of the three agar cubes. Record in your data table. 3. Calculate the surface area to volume ratio of each of the three agar cubes. Record in your data table. 4. Predict by ranking them 1st, 2nd and 3rd, which agar cubes will diffuse the fastest. Record your rankings in your data table. 5. Pour 200ml of 0.1 M sodium hydroxide solution into a 400 ml beaker. 6. Note the time and immerse the three blocks into the beaker and let them soak for 10 minutes with periodic gentle stirring and turning. 7. After 10 minutes, use a spoon or tongs to remove the blocks and blot dry with a paper towel. 8. Cut each cube in half and measure the depth of diffusion to which the pink color has penetrated. Record these measurements in mm in your data table. 9. Calculate the diffusion rate by dividing the diffusion depth by 10 minutes. Questions Part 1: 1. Which cube had the largest surface area? What problems could occur with this cell? 2. Which cube was the most efficient at diffusion? Why? 3. Give an example of why diffusion is so important in living cells. Part 2: Diffusion In this part of the lab, you will use dialysis tubing to portray a plasma membrane and see if certain substances go through it. Procedure: 1. Pour 15 ml of glucose/starch solution into a graduated cylinder. 2. Obtain a piece of dialysis tubing from the container of water. Tie a tight knot in one end of the tubing with dental floss or string. Make sure it won’t leak. 3. Open the other end of the dialysis tubing by rubbing it between your fingers. Pour the 15 ml of glucose/starch solution into the dialysis tubing. Be careful not to clog the pores of the dialysis tubing. Keep contact to a minimum. 4. Determine the initial glucose content in the tubing by dipping a glucose indicator strip into the solution. Record the data in your data table. 5. Tie a knot in the open end of the tubing. Be sure to leave space for expansion. 6. Note the color of the solution in the bag. Record in your data table. 7. Fill a 250 ml beaker 2/3 full of distilled water. Add 4 to 5 ml of IKI to the beaker. Note the color of the beaker solution and record in your data table. 8. Determine the initial glucose content in the beaker by dipping a glucose indicator strip into the beaker. Record in your data table. 9. Blot dry your dialysis tubing with paper towel. 10. Completely immerse the dialysis tubing in your beaker for 30 minutes or until there is a noticeable change in the color of the beaker or dialysis bag. If time is pressed, ask your teacher if you can add IKI. 11. Remove the dialysis bag from the beaker. Record the final color of the bag and the beaker solutions. 12. Determine the glucose content in the beaker and in the dialysis bag using the strips. To test the solution in the bag, pour the solution into a plastic dish or beaker and insert the strip. 13. Clean up your area and wash your hands. Questions: 1. Which substances were entering the bag and which are leaving the bag? What experimental evidence supports your answer? 2. Explain the results in all four boxes in your data table. Include concentration differences or membrane pore size in your discussion. 3. How could this part of the lab be modified so that our data was quantitative? 4. Rank in order by relative size, beginning with the smallest: glucose molecules, water molecules, IKI molecules, membrane pores, and starch molecules. 5. Which results would you expect if the experiment started with a glucose and IKI solution inside the bag and only starch and water outside? Why? Part 3: Osmosis In this part of the lab you will use dialysis tubing again and see the effects of placing them into different concentrations of sucrose solution. Procedure: 1. Obtain 5 pieces of dialysis tubing from a container of water. Tie a tight knot in one end of the tubing. Prevent any leaking. 2. Label the very ends of the dialysis tubing: 0M, 0.2M, 0.4M, 0.6M and 0.8M. 3. Obtain 25 ml of each sucrose solution and place into the appropriate dialysis tubing. 4. Be sure to leave space for expansion and tie a knot to prevent a leakage. 5. Blot the bags dry with paper towel. 6. Weigh each bag and record the initial mass in your data table. 7. Obtain 5 beakers and fill ¾ full with distilled water. 8. Immerse 1 dialysis bag in each beaker for 30 minutes. 9. During the 30 minutes, set up lab C. 10. After 30 minutes, remove the bags and blot them dry completely. 11. Weigh each bag and record the final mass in your data table. 12. Calculate the percent change in mass for each of the dialysis bags using the following formula: % change = (Final Mass – Initial mass) X 100 Initial Mass 13. Record % mass in your data table. 14. Obtain class averages for % change and record in your data table. 15. Graph the class average results for % change. Connect data points by a bestfit line. Questions 1. Explain the relationship between the change in mass and the molarity of sucrose within the dialysis bags. 2. Predict what would happen to the mass of the 0M, 0.2M, 0.4M, 0.6M and 0.8 M in this experiment if all the bags were placed in the 0.4M sucrose solution instead of distilled water. Explain your response. 3. Why did you calculate the percent change in mass rather than simply using the change in mass? 4. Were the sucrose solutions in the bags hypertonic or hypotonic in relation to the distilled water in the beaker? 5. A dialysis bag is filled with distilled water and then placed in a sucrose solution. The bag’s initial mass is 20g and its final mass is 18g. Calculate the percent change of mass, showing your calculations. Part 4: Water Potential In this part of the lab, you will be identifying the concentrations of sucrose solutions and then using the solutions to determine the water potential of a regular potato and a rutabaga potato. Procedure: 1. Obtain 20 pieces of potato. Obtain 5 plastic cups and label them A,B,C,D,E and label your group name. 2. Weigh 5 pieces of potato individually and record the initial mass in your data table for each potatoes. 3. Pour 30 ml of the appropriate solution into the appropriate plastic cup. Do this for all 5 cups. 4. Place 1 piece of potato into each cup. 5. Repeat steps 1-4 for rutabaga. For initial mass, record in your data table for rutabagas. 6. Place all cups in the refrigerator overnight. 7. On the next day, pour out the solutions and blot dry each potato completely. 8. Weigh each group of potatoes and record the final mass in your data table. 9. Calculate the percent change. 10. Figure out which solution belongs to 1.0M, 0.8M, 0.6M, 0.4M, 0.2M sucrose solutions! 11. Graph the results. 12. Determine the molar concentration of both potatoes. This would be the sucrose molarity in which the mass of the potato core does not change. To find this, draw a best-fit line from your data on the above graph for both potato lines. The point at which this line crosses the x-axis represents the molar concentration of sucrose with a water potential that is equal to the potato tissue water potential. At this concentration there is no net gain or loss of water from the tissue. Indicate these concentrations of sucrose in your lab notebook below your graph. Part D: Calculation of Water Potential from Experimental Data 1. What is the water potential of the potato cells? Sucrose has an ionization constant (i) of 1.0 because sucrose does not ionize in water. You will need to take the temperature inside our refrigerator to find T. Show ALL calculations in your notebook. 2. What is the water potential of the rutabaga? Again show ALL work below: 3. If the potato core were allowed to dehydrate by sitting in open air, would the water potential of the potato cells decrease or increase? Why? Figure 1.5 4. In Figure 1.5, the beaker is open to the atmosphere. What is the pressure potential of the system? 5. In Figure 1.5, where is the greatest water potential, in the beaker or in the dialysis bag? 6. So, water will diffuse __________ the bag. Why? 7. What effect does adding solute have on the solute potential component of that solution? Why? 8. Consider what would happen to a red blood cell (RBC) placed in distilled water: a. Which would have the higher concentration of water molecules, Distilled water or RBC? b. Which would have the higher water potential, distilled water or RBC? c. What would happen to the red blood cells?