Potato Diffusion Lab Handout
advertisement

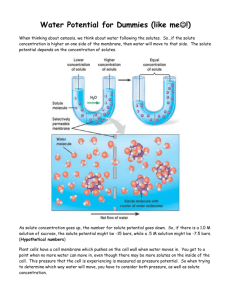
Potato Diffusion Lab Background Osmosis is a special kind of diffusion that occurs as water is separated by a selectively permeable membrane with different solute concentrations on either side of the membrane. During osmosis, water moves from regions of low solute concentration to regions of high solute concentration without the expenditure of energy. Organisms rarely exist in environments with solute concentrations that match their cytoplasm; there are usually more or fewer dissolved particles in one of two compared solutions separated by a membrane, such as a cell and the media in which it exists. A hypertonic solution is a solution in which the solute concentration is higher outside of the cell; therefore, water will flow to the external environment, causing the cell to shrink. Hypotonic solutions consist of a low concentration of solutes outside the cell; therefore, water will flow into the cell, causing cellular expansion. In the case of plant cells with cell walls, expansion is restricted, so pressure builds. This pressure is called turgor pressure. Isotonic solutions, on the other hand, are solutions in which the solute and solvent concentrations are at equilibrium: there is no net flow of materials across the selectively permeable membrane. Only a solute’s relative concentration, or water potential (Ψ), affects the rate of osmosis. Water potential consists of two components – pressure potential (ΨP ) the exertion of pressure on a solution; and solute (or osmotic) potential (ΨS), the relative concentration of solutes within the two solutions. Water Potential (Ψ) = Pressure Potential (ΨP) + Solute Potential (ΨS) Water moves from an area of high water potential (free energy) to an area of lower water potential. For example, in Figure 4 the water initially enters the tube because there is a negative solute potential in the sugar–water solution. However, the force of gravity begins to exert pressure on the rising column; when the force of gravity (pressure potential) equals the solute potential, the sugarwater solution in the column stops rising. The water potential is at zero and dynamic equilibrium has been established. The pressure potential can be determined from the height of the column. With the water potential and the pressure known, solute potential can be experimentally determined. The solute potential can be calculated as ΨS= –i CRT, where: i = ionization constant (number of ions Na+ Cl- = 2, sucrose = 1) C = Molar concentration R = pressure constant (0.0831 Liter bars/mole, ° Kelvin) T = Temperature in Kelvin (273 + temp C) bar = measure of pressure 1 bar = 1 atmosphere at sea level In this lab, you will determine the molarity of several “mystery” solutions. These solutions are different molarities of sucrose (1M, .8M, .6M, .4M, .2 M) and distilled water. You will then use the solutions to determine the water potential of potato cells. Materials Potato slice (2-3cm thick) Potato borer Triple beam balance 3 Paper cups or beakers Paper towel “Mystery” solutions Procedure 1. Pour your three assigned liquids into each cup (about 50 ml). Record the color of the solutions in your data table. 2. Obtain 9 potato cores. 3. Weigh the potatoes in groups of 3 and place them in the cups. Record the initial masses in your data table. 4. Predict the molar concentration of the potato core. 5. Let it stand overnight. 6. Remove the cores from and blot gently on a paper towel. 7. Weigh each set of cores. Record their final masses in the data table. 8. Calculate the change in mass and percent change. Data table 1: Potato Core – Group Data Solution Color Initial Mass Final Mass Mass Difference Percent (%) change in mass Data table 2: Potato Core Results – Class Data % change in Mass of Potato Cores Solution Color Group 1/5 Group 2/6 Group 3/7 Group 4/8 Clear Yellow Green Blue Pink Purple Determine which colors correspond to each molarity of sucrose: Data table 3: Identifying the Mystery Solutions Solution Color Molarity Brown Yellow Green Blue Pink Purple Class Average Percent change in mass Class Average Potato Diffusion Lab Summary Table: Color/Molarity/Percent Change in Mass (Class Average) Graph Results (Preferably computer-generated) Summary: Summarize the results of the experiment and andwer the following questions in a paragraph: Which solutions were hyper/hypo/isotonic? How do you know? What is the molarity of sucrose in the potato cells? How do you know?