Osmosis and Diffusion Lab Report: High School Biology
advertisement

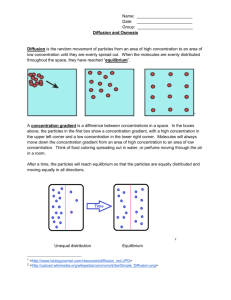
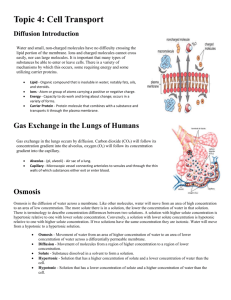
Osmosis and Diffusion Lab Introduction: The molecules that make up solids, liquids, and gases are in constant motion. They move from areas of higher concentration to areas of lower concentration by the process of diffusion. Some molecules are also able to diffuse into and out of living cells, providing a source of nutrients and allowing for the export of cell products or wastes. A cell is surrounded by a cell membrane made mostly of lipids and proteins. The membrane is selectively permeable because it allows some materials, but not others, to move across the membrane. Small ions and molecules of oxygen (O2), carbon dioxide (CO2), water (H2O) can move across the membrane freely. Other molecules, especially those that do not mix well with lipids (oily or fatty substances) and large macromolecules, must move through special pores or channels in the cell membrane made up of proteins. A dialysis tube is similar to a cell membrane in that it allows certain molecules to pass through, but keeps other molecules out. The movement of water through a selectively permeable membrane is a special type of diffusion called osmosis. Water moves from an area of lesser solute concentration (hypotonic) to an area of greater solute concentration (hypertonic). Solute Hypotonic Solution Solvent Hypotonic Hypertonic Hypertonic Membrane Generally animal cells are adapted to an isotonic environment. If placed in pure water, the water will move into the cell and the cell will expand until in bursts (cytolysis). The cell membrane is very elastic (like a balloon). Special adaptations allow some animal cells to live in hyper and hypotonic solutions. Plants generally depend on a hypotonic environment for water uptake. When placed in water, the water will move into the plant cell, but the cell wall surrounding the cell membrane is not very expandable. Pressure builds up within the cell from the influx of water. The pressure or force directed against the cell wall is called turgor pressure. If you put limp celery or a wilted flower into water, the cells will take up water and become turgid. Water molecules, in the process of osmosis never stop moving. Even when the concentration of solute is equal on both sides (isotonic), the water molecules move in and out of a cell at an equal rate. The same number of water molecules move in and out, so the system remains in equilibrium. Terms to Know: Solute – material or particles that are dissolved in a liquid (i.e. salt). Solvent – the liquid that the above material is dissolved in (i.e. water). Solution – the combination of the solute and the solvent (i.e. salt water). Hypotonic – an area of lesser solute concentration. Hypertonic – an area of higher solute concentration. Isotonic – an area of equal solute concentration. Safety: Be sure to wear goggles and an apron and do not come in contact with the iodine. Iodine toxic if swallowed, hazardous to eyes and will stain clothing. Iodine, a yellow-brown liquid, turns bluish black when mixed with starch. Also, closed toed shoes are required because we will be working with glass. Pre Lab Questions: 1. What is the difference between diffusion and osmosis? 2. How is dialysis tubing like a cell membrane? (Use the term selectively permeable in your answer.) 3. The diagrams above show hypertonic and hypotonic solutions. Draw an isotonic solution below. Solute Solution Solvent Isotonic Isotonic Membrane 4. What does it mean that the isotonic solution is at equilibrium? 5. What happens to animal cells in pure water (hypotonic solution)? 6. What happens to plant cells when they are in a hypertonic solution and loose water? 7. What happens when iodine comes into contact with starch? 8. What is one safety concern in this lab? Osmosis and Diffusion Lab Purpose: To observe how the processes of osmosis and diffusion that affect cells. Part I: Diffusion of Iodine Materials: Dialysis tubing Starch solution 250 ml beaker Iodine solution Glass stir rod Procedure: 1. 2. 3. 4. 5. 6. 7. Tie a knot in one end of the dialysis tubing. Pour starch solution into the dialysis tube, leaving enough room to tie a knot in the other end. Fill the 250 ml beaker halfway with water and add ten drops of iodine, stir. Place the dialysis tubing in the beaker with the iodine water mixture. Record your initial observations of the starch solution and the iodine solution in the data table. Wait twenty minutes and record your final observations in the data table While you are waiting, set up Part II of the lab. Part II: Osmosis in Celery Materials: Triple beam balance 2 approximately equally sized pieces of celery 2 beakers Water Graduated cylinder Salt 2 pieces of aluminum foil Tape Procedure: 1. Label each beaker with your group names. Label one beaker ‘Salt Water’ and the other ‘Fresh Water.’ 2. Put 100 mL of tap water in each beaker. Put one teaspoon of salt into the ‘Salt Water’ beaker and stir until dissolved. 3. Record the mass, length, and width (at the widest point) of one celery stick, then place it in the ‘Salt Water’ beaker. 4. Repeat step 3 for the ‘Fresh Water’ celery stick. 5. Cover each beaker with foil and leave overnight. 6. Record the mass, length, width (at the widest point) and qualitative observations of each celery stick. Data: Part I: Diffusion of Iodine Before Experiment After Experiment Observations of Starch Solution Observations of Iodine Solution Part II: Osmosis in Celery Salt Water Celery Stick Before Experiment After Experiment Difference Before Experiment After Experiment Difference Mass (g) Length (cm) Width (cm) Qualitative observations Fresh Water Celery Stick Mass (g) Length (cm) Width (cm) Qualitative observations Osmosis and Diffusion Lab Analysis Questions: Part I: Diffusion of Iodine 1. Solution A has 5% (95% water) solute. Solution B has 20% solute (80% water). These two solutions are placed on opposite sides of a semi-permeable membrane. a. Draw the solute into the diagram. Use one dot for each percent solute. b. Which side is hypertonic? c. Which side is Hypotonic? Semi-permeable Membrane Solution A (5%) Solution B (20%) 2. Draw an arrow on your diagram to show which way the water (solvent) will move. Be sure to label the arrow with the word ‘water’. 3. If we assume that the solute was able to pass through the membrane, draw a second arrow on your diagram to show direction the solute would move. Be sure to label the arrow with the word ‘solute’. Part II: Osmosis and Celery 1. Did water move into or out of the celery cells in the salt water? 2. Did water move into or out of the celery cells in the fresh water? 3. One way to prevent salad and vegetables from wilting is to cover them with plastic wrap. Why is this? (Explain in terms of osmosis.) 4. Supermarket workers spray fruits and vegetables with water to make them more desirable to consumers. Why does spraying vegetables with water prevent them from drying out? CONCLUSION QUESTIONS: Answer in paragraph form Part I: Diffusion of Iodine What do lab results indicate? o Which molecule was small enough to diffuse? How could you tell? o Which molecule was too large to diffuse? How could you tell? o BONUS: What other molecule might have diffused? Why? Part II: Osmosis and Celery What do lab results indicate? o What happens to celery in a hypotonic solution? What happens to celery in a hypertonic solution? Did the results support your hypothesis? Why or why not?