Lab 1
advertisement

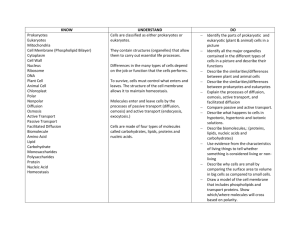
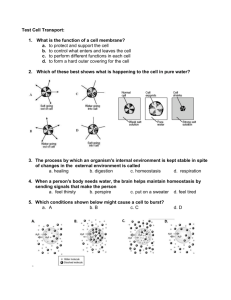
AP Biology Name _________________________________ Date START____/____ FINISH ____/____ Period _____ Score _____ Sign-Off _______ Lab Partner __________________ Table ___ Investigation 4: DIFFUSION AND OSMOSIS Big Idea 2: Cellular Processes: Energy and Communication PRELAB: Be ready to answer the following questions: (any could be found on the written portion of your exams) 1. What is kinetic energy and how does it differ from potential energy? 2. What environmental factors affect kinetic energy and diffusion? 3. Why do these factors alter diffusion rates? How do they affect rates? 4. How are gradients important in diffusion and osmosis? 5. What is the explanation for the fact that most cells are small and have cell membranes with many convolutions 6. What is the equation used to calculate water potential? (Be sure to describe the two components that are used to calculate it.) Why type of experiment could you design to identify the water potential of a carrot? (or other plant) 7. Will water move into or out of a plant cell if the cell has higher water potential than the surrounding environment? 8. What would happen if you applied saltwater to a plant? 9. How does a plant cell control its internal (turgor) pressure? PROCEDURE 1: Surface Area and Cell Size Step 1: 1. Which solution is an acid? ___________ 2. Which solution is a base? ___________ 3. What color is the dye in the base? ___________ In the acid? ___________ Step 2: Helpful equations: Surface Area: 6a2 (a = length of one side of a cube) Volume: a3 % Penetrance = Penetrated Volume (total volume minus remaining volume)/Total Volume Cut three cubes: 1cm per side, 2cm per side and 3 cm per side of the alternative colored gelatin. You will be using white vinegar in place of the 0.1M HCl Table 4.1 Date ___/___ Time ___:___ BEFORE AFTER Surface Area Total Volume Surface Area to Remaining % Penetrance Cube Sides Volume Ratio Volume 1 smallest: 2 middle: 3 largest: 1. Which block had the greatest % penetrance? ___________ 2. How do you explain the differences? PROCEDURE 2: Modeling Diffusion and Osmosis 1. Why would it be important for an IV solution to have salts in it instead of pure water? 2. How can you use weights of the filled cell models to determine the rate and direction of diffusion? AP Biology Investigation 04: 1 3. What would be the appropriate control for question #2? Steps 1-4: You will choose three pairs of solutions (instead of four) and our 4th tube will be water/water pairing. Make one of your pairings be two 1M solutions ~ with one of those solutions being 1M NaCl. Table 4.2 Date ___/___ Time ___:___ Pairing Tube Sol’n Cup Sol’n Prediction of Initial Weight Final Weight % Change tube weight (+/-) 1 2 3 4 Water Water No change 1. Which pair(s) that you tested did not have a change in weight? How can you explain this? 2. Explain the results of the pairing with the two 1M solutions. 3. Does the protein solution have a high molarity? What is evidence for your conclusion? (If you did not use albumin in your pairings, consult the data from a group that did.) 4. How could you test for the diffusion of glucose? 5. How is the dialysis tubing functionally different from a cellular membrane? PROCEDURE 3: Observing Osmosis in Living Cells 1. What would happen if you applied saltwater to the roots of a plant? Why 2. Will water move into or out of a plant cell if the cell has a higher water potential than its surrounding environment? Step1: Put a drop of distilled water on the slide, the a single leaf, then bring the edge of the cover slip at an angle to the edge of the water, slowly lower coverslip to avoid air bubbles. Before you look at the slide, record your prediction in Table 4.3 1. Where is the cell membrane in relation to the cell wall? AP Biology Investigation 04: 2 2. Can you see the two structures easily? Why or why not? 3. What parts of the cell that you see control the water concentration inside the cell? 4. What changes would you expect to see when plant cells are exposed to the solutions in Procedure #2? Step 2: Table 4.3 Date ___/___ Time ___:___ Plant Cell in Prediction Solution of: Distilled water Results Designing and Conducting Your Own Investigations: Part 1: Identify the concentrations of various sucrose solutions. There are five different colored solutions. From what you have learned so far, design and conduct an experiment to identify which color is the: 0.2M, 0.4M, 0.6M, 0.8M and 1.0M solution. Include the following in a one page typed paper: A. Hypothesis, B. Procedures (simplified), C. Data table (with the control and the independent/dependent variable(s) indicated) and D. Conclusions. You may include your name and your lab partner’s name on the typed paper, one turn-in if you complete the write-up together. SKETCH OUT YOUR GENERAL PROCEDURES BELOW: Part 2: Determine the water potential, Ψ = ΨS + ΨP, of a carrot or other plant. Strong suggestions: use the sucrose solutions above to identify the molarity (C) where the carrot’s net osmosis rate is close to zero. (I would graph the results to predict the most accurate molarity.) Use the molarity (C) identified to calculate the solute potential (ΨS=-iCRT) and ultimately the water potential. HINT: the pressure potential, ΨP, will be zero when the osmosis rate is close to zero. Your “partner” typed paper should include the same components (A-D) listed in Part 1 above. SKETCH OUT YOUR GENERAL PROCEDURES BELOW: AP Biology Investigation 04: 3