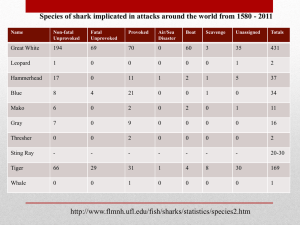
Population Dynamics of the Blue Shark, Prionace glauca, in the
advertisement

Population Dynamics of the Blue Shark, Prionace glauca, in the North Atlantic Ocean Alexandre Aires Silva A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2008 Program Authorized to Offer Degree: School of Aquatic and Fishery Sciences ©Copyright 2008 Alexandre Aires Silva University of Washington Graduate School This is to certify that I have examined this copy of a doctoral dissertation by Alexandre Aires Silva and have found that it is complete and satisfactory in all respects, and that any and all revisions required by the final examining committee have been made. Chair of the Supervisory Committee: ____________________________________________________________ Vincent F. Gallucci Reading Committee: ____________________________________________________________ Vincent F. Gallucci ____________________________________________________________ André E. Punt ____________________________________________________________ John R. Skalski Date:___________________________ In presenting this dissertation in partial fulfillment of the requirements for the doctoral degree at the University of Washington, I agree that the Library shall make its copies freely available for inspection. I further agree that extensive copying of the dissertation is allowable only for scholarly purposes, consistent with “fair use” as prescribed in the U.S. Copyright Law. Requests for copying or reproduction of this dissertation may be referred to ProQuest Information and Learning, 300 North Zeeb Road, Ann Arbor, MI 48106-1346, 1-800-521-0600, to whom the author has granted “the right to reproduce and sell (a) copies of the manuscript in microform and/or (b) printed copies of the manuscript made from microform.” Signature_______________________________ Date___________________________________ University of Washington Abstract Population Dynamics of the Blue Shark, Prionace glauca, in the North Atlantic Ocean Alexandre Aires Silva Chair of the Supervisory Committee: Professor Vincent F. Gallucci School of Aquatic and Fishery Sciences Large numbers of blue sharks are caught as bycatch, and have even become the target species in pelagic longline fisheries in the North Atlantic Ocean. The status of the stock is ambiguous due to the limitations of the fishery-dependent and -independent data. In chapter 2, fishery-independent demographic and risk analyses are developed for the North Atlantic blue shark. An age-structured matrix population model in which the life-history parameters are stochastic was build. A risk analysis to evaluate the future prospects of the population under different harvest rates is presented and implications for management discussed. Chapter 3 develops a historic index of abundance for the blue shark in the western North Atlantic. Pelagic longline catch records from several fishery observer programs that operated since the late 1970s are linked to observations from historical fishery-independent longline surveys undertaken in the late 1950s, 1960s and 1970s. Generalized linear model (GLM) techniques are used to derive standardize catch rates (catch-per-unit-effort, CPUE). The index of abundance revealed a decline in blue shark CPUE of approximately 30% in the western North Atlantic from 1957 through 2000. The magnitude of this decline is less than other recently published estimates and seems reasonable in light of the high productivity of blue shark revealed by the demographic analyses in Chapter 2. Chapter 4 analyzed the available tagging data in a spatio-temporal analysis to derive estimates of movement and fishing mortality rates for the blue shark in the North Atlantic Ocean. The analyses are based upon the historical blue shark tagrecovery data archived by the U.S.-NMFS Cooperative Shark Tagging Program (1965-2004). Fishing mortality rates (F) were highly heterogeneous across the North Atlantic Ocean. Estimates of F for the western North Atlantic were historically lower than 0.10 yr-1, well below a critical reference point, Fc (=0.20 yr-1), derived from the risk analysis in Chapter 2. Such low fishing mortality rates are consistent with the low declines in catch rate estimated for the western North Atlantic in Chapter 3. In contrast, F estimates over the most recent decade (1990s) in the eastern side of the Atlantic were found to be rapidly approaching Fc . TABLE OF CONTENTS Page List of Figures ............................................................................................................. iii List of Tables .............................................................................................................. iv Chapter 1: Background and Synopsis ............................................................................1 1.1. The Context-of-the-Problem .......................................................................1 1.2. The Case Study – North Atlantic Blue Shark .............................................4 1.2.1. The Single Stock Concept ............................................................4 1.2.2. The Fishery Exploitation Scenario...............................................5 1.2.3. Knowledge About Stock Status ...................................................9 1.3. Dissertation Outline ..................................................................................10 Chapter 2: Demographic and Risk Analyses Applied to Management and Conservation of the Blue Shark in the North Atlantic Ocean ................................14 2.1. Introduction ...............................................................................................14 2.2. Materials and Methods ..............................................................................18 2.2.1. Age-Structured Model ...............................................................18 2.2.2. Uncertainty of Life-history Parameters .....................................20 2.2.3. Stochastic Demographic Analysis .............................................22 2.3.4. Population Projections and Risk Analysis .................................25 2.3. Results .......................................................................................................27 2.3.1. Survivorship ...............................................................................27 2.3.2. Demographic Analysis ...............................................................28 2.3.3. Risk Analysis .............................................................................30 2.3. Discussion .................................................................................................31 i Chapter 3: A Historic Index of Abundance for the Blue Shark in the Western North Atlantic ........................................................................................................47 3.1. Introduction ...............................................................................................47 3.2. Materials and Methods ..............................................................................51 3.2.1. Data Selection ............................................................................51 3.2.2. Catch-Effort Standardization .....................................................53 3.3. Results .......................................................................................................59 3.3.1. Model Selection .........................................................................59 3.3.2. Catch-Effort Standardization .....................................................60 3.4. Discussion .................................................................................................62 Chapter 4: A Spatially-Structured Tagging Model to Estimate Movement and Fishing Mortality Rates for the Blue Shark in the North Atlantic Ocean ..............80 4.1. Introduction ...............................................................................................80 4.2. Materials and Methods ..............................................................................83 4.2.1. Tagging Data ..............................................................................83 4.2.2. Geographical Stratification and Definition of Fisheries ............84 4.2.3. Model Development...................................................................85 4.2.4. Model Hypotheses – Base Case Model and Sensitivity Analyses ........................................................................................91 4.3. Results .......................................................................................................93 4.3.1. Model fit to Observed Tag Recoveries ......................................93 4.3.2. Movement Parameters ...............................................................94 4.3.3. Fishing Mortality Rates..............................................................94 4.4. Discussion .................................................................................................97 Bibliography ..............................................................................................................119 Appendix A: Supplementary Information of Chapter 2 ............................................137 Appendix B: Supplementary Information of Chapter 3 .............................................141 Appendix C: Supplementary Information of Chapter 4 .............................................151 ii LIST OF FIGURES Figure Number Page 2.1. Uncertainty in the Life-History Parameters of the North Atlantic blue Shark ................................................................................................................42 2.2. Statistical Distributions of Demographic Parameters for an Unfished Blue Shark Population ..............................................................................................43 2.3. a) Isoclines for the Average Response in Population Growth Rate to Different Harvest Rates Starting at Different Ages-at-First-Capture. b) Distributions Derived for the Stationary Harvest Rate ................................44 2.4. Example Stochastic Population Projections....................................................45 2.5. Risk Curves for Depleting the Population to Levels Below 50% of its Pre-Exploited Population Size .........................................................................46 3.1. Geographical Locations of Pelagic Longline Sets for Each Target Species Fishing Operation.............................................................................................76 3.2. Diagnostic Check Plots for the Different Error Models Fit to the Blue Shark Longline Reconciled Data Set ...............................................................77 3.3. Delta-Lognormal Model Estimates .................................................................78 3.4. Mean Effects of Selected Explanatory Factors on Blue Shark Catch Rates ...79 4.1. Blue Shark Tagging Data Collected by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (1965-2004). .........................113 4.2. Annual Time Series of Blue Shark Tag Releases and Recoveries in each Region of the North Atlantic Ocean. .............................................................114 4.3. Movement Hypothesis for the Blue Shark in the North Atlantic Ocean Assumed in the Tagging Model. ....................................................................115 4.4. Base Case Model Fit to the Blue Shark Tag Recovery Observations for each Fishery by Geographical Region. ..........................................................116 4.5. Fishery-Specific Instantaneous Fishing Mortality Rates in Each Geographical Region as Estimated from the Base Case Model. ...................117 4.6. Sensitivity Analyses of the Estimate of Total Instantaneous Fishing Mortality to Different Scenarios About the Tag Shedding Rate and the Tag Reporting Rates of Major Fisheries ...............................................................118 iii LIST OF TABLES Table Number Page 2.1. Definition of Mathematical Notation ..............................................................38 2.2. Estimates of Natural Survivorship Produced by the Indirect Methods...........39 2.3. Descriptive Statistics for the Demographic Parameters..................................40 2.4. Risk (Probability) of Obtaining a Population Decline to Levels Below 50% of the Pre-Exploited Levels .....................................................................41 3.1. Fishery-Independent and –Dependent Pelagic Longline Data Sources ..........70 3.2. Summary Statistics of Fishery-Independent and Fishery-Dependent Data ....71 3.3. Annual Time Series of Pelagic Longline Sets for the Different Target Species .............................................................................................................72 3.4. Categorical Variables Assumed in the Blue Catch-Effort Standardization ....73 3.5. Summary Statistics of the Selected Four Error Models ..................................74 3.6. Delta-Lognormal Standardized CPUE for the Blue Shark .............................75 4.1. Blue Shark Tag Recoveries Reported by Fishing Fleet (Nation or Group) for Each Geographical Region .......................................................................107 4.2. List of the Fisheries Defined in the Tagging Model .....................................108 4.3. Estimated Parameters of the Tagging Model. ...............................................109 4.4. Model Sensitivity Analysis. ..........................................................................110 4.5. Movement and Fishing Mortality Parameter Estimates and their Coefficient of Variation (CV) Obtained from the Base Case Model.............111 4.6. Catchability Parameter Estimates Obtained for each Fishery from the Base Case Model............................................................................................112 iv ACKNOWLEDGEMENTS I learned early on along the way that “nobody does it alone” in most important endeavors of life. This is also valid for the scientific and scholarly process. I am forever grateful for the encouragement, support, friendship and kindness of many friends and colleagues with whom I had the privilege to cross my path with during my voyage as a doctoral student at the University of Washington. My graduate program was made possible through several sources of financial support. I am eternally grateful to my main sponsors: the Fulbright Commission and the Fundação Luso-Americana para o Desenvolvimento in Portugal. I also extend my gratitude to my following supplementary sponsors at the University of Washington: the Graduate School, the Claire L. and Evelyn S. Egtvedt Fellowship, the Galen and Helen Maxfield Fisheries Scholarship, and a Wakefield Scholarship fund. My special thanks to Jean Whitcomb at the Graduate School for the support over the years. I wish to express my most sincere gratitude to my major advisor, Dr. Vincent F. Gallucci for his wise mentorship during my graduate program. By pointing me into the right direction, Vince’s advice was critical to “convert” me from a field fisheries biologist into a quantitative fisheries sock assessment scientist. Vince helped to transform my fascination on the blue shark problem, which I acquired in the waters of Portugal, into a real dissertation project. By allowing me enough space to explore my own research interests, Vince helped me to become an independent scientist without losing focus on the importance of team work. I thank Vince for the countless overnight hours put into critically reviewing my manuscripts and helping to make them suitable for publication. Because of that, all the chapters presented in this dissertation will truly become disseminated, rather than to eternally lie in the archives of a library. His constant support, advice and guidance played a key role in my success. I deeply appreciate our strong friendship. Besides my major advisor, I am deeply grateful to the five additional members of my supervisory committee. I am particularly indebted to Drs. André Punt and John Skalski for their permanent support, their critical review of my work and manuscripts, and for the intellectual challenge along my program. My sincere thanks to Dr. Miles Logsdon for helping me to find a bit of space for the tools of Geographical Information Systems (GIS) in my research, and for broadening my interests on spatial analysis. I thank Miles for his encouragement and patience. Dr. Gregor Cailliet (MLML-CSU) has inspired my shark research since I was an undergraduate student in Portugal. It was a privilege to have Greg on my committee. My special thanks to Greg Cailliet for inspiring me not to loose focus of the real biological problems in the midst of mathematical sophistication. I am honored to have had Dr. Jerry Franklyn assigned as the Graduate School Representative to my committee. My hearty v gratitude to Dr. Franklin for his prompt availability and for his expressed interest at committee meetings. Although not an official member on my supervisory committee, Dr. John Hoey (NOAA-NEFSC) ex-officio’s role on the committee is totality legitimate. I am eternally grateful to John Hoey for his constant mentorship, inspiration and guidance. John made it possible for my research experience to physically spread out from SAFS-UW, Seattle, WA, into two labs on the east coast (NOAA’s headquarters in Silver Spring, MD, and then the NMFS-NEFSC Narragansett laboratory, Narragansett, RI). John showed me that a deep understanding of fisheries data, as well as the fisheries reality form which it was sampled from, should precede analysis. Our journey together through the recovery and construction of the longline historical database was fascinating. I greatly benefited from John’s extensive knowledge about the pelagic longline fishery in the North Atlantic. I am forever grateful to Maria and John Hoey for truly embracing me as an additional family member during many trips to the NMFS Narragansett lab, Rhode Island. I deeply value our friendship. My fascination for the North Atlantic blue shark program initiated through my exposure to the tagging results of the U.S. NMFS-Cooperative Shark Tagging Program back in Portugal. Later on, it was a privilege to get fully immersed into the program during many working trips to Rhode Island. My profound gratitude for the support and friendship that I found at the Narragansett laboratory, RI. I am particularly indebted to John Hoey and Nancy Kohler for allowing me to pursue this project. My heartfelt thanks go to Patricia Turner, Ruth Briggs, Kelly Kreis for their invaluable support with the tagging and survey data. I am also grateful to Chris Orphanides for his support with GIS. I thank Harold “Wes” Pratt, Lisa Natanson, Karen Tougas, and Anthony Wood for their support. I am immensely grateful to Cami and Steve McCandless, as well as to Ruth Briggs for kindly receiving me at their homes. I thank David Martins and Amy VanAtten for their friendship, and the wonderful family moments under a severe winter blizzard on the east coast. A summer working trip to NOAA’s headquarters in Silver Spring, MD, was also part of my research. My thanks to Mark Holliday and Liz Pritchard (NOAA), and to Fred Moreno for the great hospitality in Silver Spring. I extend my gratitude to all the SAFS-UW community. A substantial amount of my training at UW came through interactions with many colleagues and friends at UW. It was a privilege to share an office with Ian Taylor at SAFS. I am immensely grateful to Ian for his permanent support, encouragement, friendship and continuous inter-change of ideas. My hearty gratitude to many other colleagues and friends at the Shark Lab for several years of camaraderie and sharing of knowledge: Joel Rice, Muktha Menon, Hans Nesse, Nicci Vega, Brian Langseth, Cindy Tribuzio, Jason Gasper, Danny Badger and Shannon O’Brien. I thank many colleagues at the Hilborn and Punt labs for support and many discussions. My special thanks to Jason Cope, Billy Ernst, Arni Magnusson, Carolina Minte Vera, Juan Valero and Ian Stewart. vi My life in Seattle was enriched by a wonderful group of friends with whom I shared this journey. I am eternally grateful to Manu Esteve and Mari Carmen (and Mafi), Juan Valero, Billy Ernst, Jesus Jurado Molina e Tina Campos, Alexandre Zerbini, Carlos Alvarez-Flores, Julian Burgos, Nancy Guarderas, Marina Roldan, Georgeana Mezerani, Carolina Minte-Vera, Manoel Souza and Nicolas Gutierrez. I thank Dan Jackson for his kind hospitality upon my arrival in Seattle. My special gratitude to Allison Claussen. My sincere gratitude to the staff of the Inter-American Tropical Tuna Commission (IATTC) in La Jolla, California. My special thanks to Drs. Rick Deriso, Mark Maunder, Guillermo Compéan and Robin Allen, for giving me enough flexibility to finish this dissertation while starting a new job at the IATTC. I am gratefully indebted to Mark Maunder for his support with the tagging analysis and for his encouragement. My journey as graduate student in the U.S. would not have started without the encouragement and support of many friends and colleagues in Portugal. My special thanks to Karim Erzini (Universidade do Algarve) and Helder Marques da Silva (Universidade dos Açores) for encouraging me to pursue a graduate program in the U.S. I extend my gratitude to all colleagues at Departamento de Oceanografia e Pescas, Universidade dos Açores (DOP-UAç). I thank Pedro Afonso, Telmo Gomes, Jorge Fontes, Valentina Costa, Ricardo Serrão Santos, Gui Menezes, Mário Rui Pinho, Ana Martins and Helen Martins. My special thanks to Sara Magalhães. I am eternally grateful to my family. To my parents, Alice (Lita) and José, to whom I dedicate this study, for allowing me to pursue my interests and dreams, even when these went beyond geographical boundaries. I am thankful for their unconditional support and love. To my brother Frederico (Nico), for his support, and for his tolerance with my physical absence in the family. Last, but not the least, I am deeply grateful to Cynthia Dai, my soon-to-be wife. My journey would not have been the same without Cynthia’s support, encouragement and love. I thank Cynthia for her patience and for helping me to see what really matters in the most challenging moments. vii DEDICATION Para a Lita, José e Nico viii 1 CHAPTER 1 BACKGROUND AND SYNOPSIS 1.1. THE CONTEXT-OF-THE PROBLEM There is great concern about the population depletions of the apex fish predators and the impacts that harvest removals can have on marine ecosystems (Pauly et al. 1998; Stevens et al. 2000; 2003). One study indicated that the biomass of the large pelagic fish predators in the world’s oceans, mainly tuna and billfish, has been reduced to 10% of their historic virgin levels (Myers and Worm 2003). These concerns extended to shark populations in various regions of the globe, producing studies that likewise indicated serious declines due to fishing pressure (Baum et al. 2003; Baum and Myers 2004; Myers and Worm 2005; Shepherd and Myers 2005; Ward and Myers 2005). The validity of these alarming prospects is subject to debate (Walters 2003; Burgess et al. 2005a; Burgess et al. 2005b; Maunder et al. 2006; Polacheck 2006). However, there is no doubt about the relatively higher vulnerability to overexploitation of certain groups of large pelagic fish predators. This is the case of certain cartilaginous fish (class Chondrichthyes), particularly the elasmobranchs (subclass Elasmobranchii) which include the sharks and rays (Compagno 1999). In most cases, sharks lie on the K side of the life-history continuum defined by r/K selection theory (Musick 1999; Stevens 1999). Their life-history strategy is usually characterized by slow growth rates over long life spans, a late sexual maturity with 2 production of limited offspring (low fecundities) after long gestation periods (Branstetter 1990; Stevens et al. 2000). Despite the lack of empirical data on the nature of the relationship between stock and recruitment (S-R) for sharks (Walker 1998; Cortés 2004), and based on their reproductive limitations, the assumption of a virtually direct S-R relationship was early on taken as a paradigm in elasmobranch fishery science (Holden 1973). This relationship implies that shark populations decline more rapidly and compensate less strongly for losses due to harvest, when compared with traditional exploited teleost fish stocks (Stevens et al. 2000). Overall, the life-strategy of sharks is similar to that evolved by marine mammals and reptiles, many of which under conservation threat (Hoenig and Gruber 1990; Musick et al. 2000). The history of shark fisheries demonstrate the general vulnerability of sharks and rays to fishery exploitation (Holden 1974; Holden 1977; Anderson 1990; Hoff and Musick 1990; Walker 1998; Cortés 2004). Typically, the exploitation begins with a highly productive phase which is shortly followed by a rapid decline in the catch rates and eventual collapse of the fishery. The following are the “classic” case study examples of this so-called “boom and bust” shark fishery pattern: the Californian fisheries for soupfin and thresher sharks (Ripley 1946; Cailliet and Bedford 1983), the Australian school shark (Olsen 1959), the basking shark of the west coast of Ireland (Parker and Stott 1965), the spiny dogfish fisheries in eastern North Atlantic (Holden 1968) and the Pacific coast of North America (Ketchen 1969; Wood et al. 3 1979), and the porbeagle fisheries in the western North Atlantic (Casey et al. 1978; Campana et al. 2002). Although the shark reproductive limitations and the numerous cases of overexploitation are not encouraging, sustainable shark fisheries may well be possible. In fact, both Holden (1973) and Walker (1998) responded positively to the question “ can shark resources be harvested sustainably?”. The limited opportunities seem to be dependent on the biology of the species and capacity for density-dependent regulation. Walker’s (1998) view goes even further when claiming that the same life-history traits that make elasmobranchs so vulnerable to exploitation can be turned into an advantage to achieve stable shark populations and sustainable exploitation patterns. This results from their high survival rates and low recruitment variability when compared to teleost species. Unfortunately, the global scenario for shark fisheries shows little signs of sustainability. Most of the world’s catches of sharks are taken incidentally (bycatch) on various fishing gears in a wide variety of non-industrial and industrial non-shark target fisheries (see overviews by Compagno 1990; Bonfil 1994; Rose 1996; Walker 1998). This non-target peculiarity, in addition to the low economic value of shark catches, has resulted in a general low priority for data collection and research by national government bodies (Stevens et al. 2000). Shark fisheries are therefore the typical example of a data-limited situation in terms of fishery-dependent statistics (Bonfil 1994). As a result, stock assessment analysis and evaluation of stock status is a difficult task for sharks. Despite the data shortcomings, preliminary evaluations of 4 the world’s shark fisheries have been made and these indicated severe declines (Castro et al. 1999). 1.2. THE CASE STUDY - NORTH ATLANTIC BLUE SHARK 1.2.1. The Single Stock Concept The blue shark, Prionace glauca Linnaeus 1758 (Carcharhinidae) is considered the most widespread and abundant of the pelagic sharks in the world’s oceans (Gubanov and Grigor'yev 1975; Compagno 1984; Nakano and Seki 2003). However, the blue shark stock structure in different oceans of the globe remains unclear since a comprehensive global genetic study has not been done. In the North Atlantic, this knowledge gap has been partially narrowed via several tagging programs (e.g., Stevens 1976; Kohler et al. 2002; Fitzmaurice et al. 2005). Tag-recovery records have shown seasonal latitudinal movements of blue sharks in both sides of the North Atlantic (e.g., Stevens 1976; Queiroz et al., 2004, for the eastern side; Casey 1982, 1985; Kohler et al. 2002 for the western side). Transatlantic migrations of blue sharks in both directions are common, but movements across the equator are extremely rare. This highly migratory and trans-boundary nature of the species became the supporting basis for the hypothesis of a single stock of blue sharks in the North Atlantic Ocean, for stock assessment and management purposes (Casey 1982; Kohler et al. 2002). Apparently, blue sharks seem to use the main North Atlantic current systems to move over the large ocean basin with different segments of the stock apparently 5 segregating by life-stage (size) and sex. While the occurrence of subadult females and adult males seems to be more prevalent in the waters of the western North Atlantic (Pratt 1979; Casey 1985; Kohler et al. 2002), neonates, juveniles and adult females are dominant on the eastern side (Castro and Mejuto 1995; Mejuto and García-Cortés 2005; Litvinov 2006; Aires-da-Silva et al. 2008a). 1.2.2. The Fishery Exploitation Scenario The blue shark meat has historically been regarded as unpalatable due to its soft texture and strong odor of ammonia (Walker 1998; Castro et al., 1999; personal observations of the author in the Azores, Portugal). In addition, its skin was considered of poor quality for the fish leather tanning industry (Draganik and Pelczarski 1984). This situation, however, has changed recently as shown by a study of the U.S trade of fish leather in which blue and tiger sharks were the dominant species among sharks (Grey et al. 2006). A major threat to blue sharks is a great demand for their fins for shark fin soup and other dishes in some Asian cultures (Draganik and Pelczarski 1984). In fact, a recent study showed that the blue shark fins dominate the world’s largest Hong Kong shark fin market (Clarke et al. 2006a). As the new technologies of blast-freezing become available to the high seas fisheries, new markets for blue shark meat as food are also emerging (Fleming and Papageorgiou 1996; Fordham 2006). 6 In the North Atlantic Ocean, blue sharks are predominantly caught as bycatch of pelagic longline fisheries targeting tuna and swordfish (Anonymous 2005). The main fisheries are described below. The Asian Far Seas Tuna Fisheries – Pelagic sharks are an important bycatch in the Asian fisheries targeting tuna in the high seas (Bonfil 1994). The major Asian nations fishing in the Atlantic Ocean are Japan, the Republic of Korea, and Taiwan. Japanese longliners have fished for tuna in the Atlantic Ocean since the mid-1950s. The fishery initially developed in the equatorial grounds of the western Atlantic and expanded to the entire ocean in 1970 (Bonfil 1994). Estimated catch rates of pelagic sharks caught by Japanese longliners in the Atlantic Ocean ranged between 1 to 10 sharks/1000 hooks (Bonfil 1994). A preliminary estimate of approximately 26, 322 metric tons was obtained for the total pelagic shark bycatch taken by the Japanese longliners in the Atlantic during 1989 (Bonfil 1994). Hoff and Musick (1990) reported that the blue shark bycatch comprised 85% of the pelagic shark bycatch taken by Japanese longliners operating in U.S. waters during the mid 1980s. The majority of the sharks caught in the Atlantic are discarded at sea after the fins are removed (Nakano 1993), since these are highly profitable in the Asian shark fin markets (Clarke et al. 2006a). Longliners from the Republic of Korea have targeted tuna in the Atlantic Ocean since the mid-1960s. The majority of the fishing effort by the Korean fleet has 7 taken place in the equatorial region between 20º N and 20º S, but mostly south of the equator (Anonymous 2007). Reports on pelagic shark catches by Korea are not available, but there is an estimate of approximately 190,000 sharks caught by Korean longliners in the Atlantic during 1989, of which around 45% were blue sharks (Bonfil 1994). It was also reported that around 97% of the pelagic sharks caught by Korean longliners in the Atlantic were discarded. However, the extent of fining practices by the Korean fleet is unknown. The Iberian Swordfish Fisheries - Blue sharks and other pelagic sharks are taken as bycatch in some European fisheries (see Heessen et al., 2003 for a review). The swordfish longline fisheries of the Iberian nations (Spain and Portugal) are the dominant sources of blue shark fishing mortality by the European fisheries. The Spanish fishery initiated its expansion throughout the waters of the eastern North Atlantic during the mid-1960s (Garcés and Rey 1988; Rey et al. 1988). This fleet was confined to the fishing grounds and archipelagos around the Iberian Peninsula and the coast of Morocco until the 1970s. Garcés and Rey (1983) estimated that six blue sharks were caught for each swordfish taken during the period from 1973 to 1981. By the mid 1980s, the fishing grounds used by the Spanish fleet expanded westward to areas close to the Grand Banks (Hoey et al. 1988; Mejuto and Iglesias 1988; Rey et al. 1988), and as far south to the Gulf of Guinea along the coast of Africa. Most of the blue shark bycatch by the Spanish fleet was discarded during the mid to late 1980s 8 (68-81%). However, fining and use of the carcass for bait were common during this period (Mejuto 1985; Rey et al. 1988). The swordfish longline fishery of mainland Portugal represents another important source of blue shark bycatch in the eastern North Atlantic since the mid 1980s (Neves-dos-Santos et al. 2002). This fishery takes place mainly in the waters around the Azores archipelago but also expands to southern latitudes off the coast of Africa (Aires-da-Silva and Pereira 1999). On average, blue sharks represented up to 86% of the total catch on a longline set by the Portuguese fishery from 1993 to 1998 (Aires-da-Silva et al. 2008a). Blue sharks are also caught as bycatch of a regional small-scale swordfish longline fishery in the Azores (Simões 1999). The North American Fisheries -Swordfish fishing by U.S. and Canadian longliners initiated in the early 1960s following reports of the incidental capture of the species by Japanese and Norwegian longliners fishing for tuna and porbeagle sharks, respectively (Anderson 1985). A westward expansion of the Spanish swordfish fleet to the fishing grounds exploited by the North American and the Japanese fisheries occurred in the 1980s (Mejuto and Iglesias 1988). The Recreational Fisheries - Pelagic sharks and mainly blue sharks are subject to recreational fishing on both sides of the North Atlantic Ocean. In the western North Atlantic, blue sharks are the main target species of U.S. and Canadian recreational fisheries (Kohler et al. 1998; Campana et al. 2005). In the eastern North 9 Atlantic, blue sharks are the main target species of recreational fisheries in waters of England, Ireland, Spain and Portugal (Heessen 2003; Fitzmaurice et al. 2005). 1.2.3. Knowledge About Stock Status An evaluation of the stock status for blue sharks is strongly handicapped by limited fishery statistics as it is for any other bycaught shark species (Bonfil 1994). Under- or non-reporting of bycatch, unknown discard levels, status (dead or alive) of discards, and poor knowledge on the extent of fining practices are among the major reasons for the lack of data. Data collection and evaluation of the stock status of the blue shark stocks in the Atlantic Ocean is conducted under the auspices of the International Commission for the Conservation of Atlantic Tuna (ICCAT). A Sub-Committee on By-catch was established in 1996 to coordinate these tasks (Kebe et al. 2002). At that time, it was still not possible to conduct stock assessments for blue shark due to the limitations of the fishery statistics. Despite continuing data shortcomings, ICCAT has recently been able to conduct assessments for the North and South Atlantic blue sharks stocks (Anonymous 2005). All of the analyses indicated that the current fishing mortality rates for the blue shark in the North and South Atlantic are sustainable, and that current biomass levels may even be close to the virgin level. These results are similar to those from stock assessments for blue shark in the North Pacific Ocean (Kleiber et al. 2001; Sibert et al. 2006). However, ICCAT considered the Atlantic assessments to be “very 10 preliminary” due to the uncertainties of the fishery statistics, and recommended better use of other sources of data available, including tagging data (Aires-da-Silva et al. 2005). The use of fishery-independent data, however, provided a different interpretation about the status of blue shark stocks worldwide. Specifically, a recent analysis of shark fin trade records indicated that blue sharks globally are being exploited at levels close to or even above maximum sustainable yield (MSY) (Clarke et al. 2006b). Unfortunately, a fishery-independent evaluation of the status of the stocks for each ocean basin is not available yet. 1.3. DISSERTATION OUTLINE The broad goal of this dissertation is to improve the quality of the scientific information available for management and conservation of the North Atlantic blue shark. The limited quality of the fishery statistics available leads to uncertainties about any blue shark stock assessment (Anonymous 2005). While improvements need to be made at the fishery-dependent data level, fishery-independent approaches are particularly needed to provide alternative (and supplemental) information to those which can be derived from traditional stock assessment techniques. The title of this dissertation should be interpreted broadly. “Population dynamics” in traditional fishery science is usually interpreted as the study of the factors that affect growth, stability, and decline of populations. This dissertation focuses on the study of three factors that affect the blue shark population dynamics: 11 demography, fishery exploitation, and movement. While independent per se, the following three chapters address one or more of these aspects and are all related. The chapters were prepared for direct submission of articles to peer review scientific journals. Supplemental information (tables and figures) not to be published are presented in the appendices. Chapter 2 presents fishery-independent demographic and risk analyses for the North Atlantic blue shark. While the fishery statistics for the blue shark are limited, it is also data-rich with respect to the availability of life-history information. An age-structured matrix population model where the life-history parameters are stochastic was built. The demography of the blue shark is investigated for both virgin (unharvested) and harvested scenarios. The results confirm that the blue shark is one of the most productive of the pelagic sharks. However, an elasticity analysis showed that this unusual capacity for population growth is dependent on the survival of juvenile blue sharks. A risk analysis to evaluate the future prospects of the population under different harvest rates is presented and implications for management discussed. The results could be used for precautionary management, as a supplement to the data-limited preliminary stock assessments. Chapter 3 develops an index of abundance that covers the largest possible period of the exploitation history of blue sharks in the western North Atlantic. This chapter aims to gain perspective on the “missing baseline” when stock conditions should have been close to pristine relative to the levels in more recent decades. Pelagic longline catch records from several fishery observer programs that operated 12 between the late 1970s and the present are linked to observations from historical fishery-independent longline surveys undertaken in the late 1950s, 1960s and 1970s. Generalized linear model (GLM) techniques are used to derive standardize catch rates (catch-per-unit-effort, CPUE) over four decades of exploitation history (1957-2000). The index of abundance revealed a decline in blue shark CPUE of approximately 30% in the western North Atlantic from 1957 through 2000. The magnitude of this decline is less than other recently published estimates and seems reasonable in light of the high productivity of blue shark revealed by the demography analyses (Chapter 2; Aires-da-Silva and Gallucci 2007). The index of relative abundance could improve the quality of the tuning process of future stock assessment models. Chapter 4 analyzed the available tagging data in a spatio-temporal analysis to derive estimates of movement and fishing mortality rates for the blue shark in the North Atlantic Ocean. Although severely limited in terms of fishery-dependent statistics, there is more tagging data for the North Atlantic blue shark than for any pelagic shark stock. The analyses are based upon the historical blue shark tag-recovery data archived by the U.S.-NMFS Cooperative Shark Tagging Program (1965-2004). Four broad geographical regions (two on each side of the ocean) were defined. Despite substantial annual mixing of blue sharks among regions, a high proportion of sharks remain in most regions each year. This spatial structure should be incorporated into North Atlantic blue shark stock assessments. Fishing mortality rates (F) were highly heterogeneous across the North Atlantic Ocean. Estimates of F for the western 13 North Atlantic were historically lower than 0.10 yr-1. Such low fishing mortality rates are consistent with the low declines in catch rates estimated for the western North Atlantic in Chapter 3. In contrast, F estimates over the most recent decade (1990s) in the eastern side of the Atlantic were found to be rapidly approaching an estimated reference point for conservation ( Fc =0.20 yr-1), estimated from the demographic and risk analyses (Chapter 2; Aires-da-Silva and Gallucci 2007). These results suggest caution since the juvenile and pregnant female segments of the stock are highly vulnerable to the fishery in the waters of the eastern North Atlantic. 14 CHAPTER 2 DEMOGRAPHIC AND RISK ANALYSES APPLIED TO MANAGEMENT AND CONSERVATION OF THE BLUE SHARK IN THE NORTH ATLANTIC OCEAN Equation Chapter 2 Section 1 2.1. INTRODUCTION There is great concern about the population depletions of large pelagic predatory fishes. A recent estimate indicates that the current biomass of many tuna and billfish stocks is only at about 10% of their pristine levels in the world’s oceans (Myers and Worm 2003). Similar alarming concerns have also been extended to shark populations from many regions of the globe (Baum and Myers 2004; Myers and Worm 2005; Ward and Myers 2005). The fin trade is one of the major threats to shark populations. A recent analysis of fin trade records showed the blue shark, Prionace glauca (Carcharhinidae), as the dominant species in the world’s largest shark fin market (Clarke et al. 2006a). The blue shark is an oceanic species with a wide-ranging distribution throughout the oceans of the globe (Nakano and Seki 2003). A second analysis of trade records suggests that blue sharks globally are currently exploited at levels close to or even above maximum sustainable yield (Clarke et al. 2006b). However, no evaluations of the blue shark stock status were made for individual ocean basins. In the North Atlantic, tag-recapture records have shown size and sex-related latitudinal movements of blue sharks in both the western side (Casey 1985; Kohler et 15 al. 2002) and the eastern side (Stevens 1976; Fitzmaurice et al. 2005; Mejuto et al. 2005). Although long-distance trans-Atlantic migrations are commonly observed in the different tagging programs, little mixing across the equator has been recorded. This tagging information, together with catch and biological data, suggest the existence of a single stock of blue sharks in the North Atlantic Ocean (Casey 1985; Kohler et al. 2002). Blue sharks are caught primarily as bycatch in tuna and billfish longline fisheries by fleets from several nations fishing in the North Atlantic (Heessen 2003; Anonymous 2005). Unfortunately, many of the logbooks for these various fleets that are available are incomplete with respect to captures and release status (alive vs. dead) of blue sharks. Limited numbers of observer programs cover only a small fraction of the commercial fleets and extend over short temporal spans. This data-limited situation handicaps a blue shark stock assessment analysis, which would be difficult enough for this highly migratory species even if complete data were available. The modeling process is complicated because of the complex sexual and life-stage segregation patterns of the blue shark (Kohler et al. 2002). Knowledge of the stock status of the blue shark in the North Atlantic is both limited and contradictory. There are two opposing views. First, Baum et al. (2003) reported the “collapse” of shark populations in the Northwest Atlantic based upon catch rate analysis. Blue sharks, in particular, are noted to have declined dramatically (by 60%) since the mid eighties. However, the methods and claims of Baum et al. (2003) have been challenged (Baum et al. 2005; Burgess et al. 2005b; Burgess et al. 16 2005a). In contrast to the view of Baum et al. (2003), an evaluation of the stock status by the International Commission for Conservation of Atlantic Tuna (ICCAT) reported that the current exploitation levels are sustainable (Anonymous 2005), and that biomass levels may even be close to the unfished carrying capacity. The assessment, however, was considered by ICCAT as “very preliminary” due to the poor quality of the data. Although the catch levels of blue sharks appear to be on the rise (Anonymous 2005; Campana et al. 2006), the dichotomous perspectives on the stock status have contributed to a low priority for management. New markets are emerging and moving the blue shark trade from one dominated by fins (Clarke et al. 2006a), to one where meat is also marketed. The new pattern is seen in the longline swordfish fisheries from Spain and Portugal, at least, where blue shark meat is a large proportion of the landings (Mejuto et al. 2002; Aires-da-Silva et al. 2008a), entering into some European markets (Fleming and Papageorgiou 1996; Fordham 2006). Management of these fisheries in the eastern North Atlantic waters is crucial since these waters include important nursery grounds where pregnant females and juveniles are particularly vulnerable (Casey 1985; Castro and Mejuto 1995). Acquisition and maintenance of a fishery-dependent database that adequately represents both exploitation by the multi-nation fleet and the spatial complexity of the stock is difficult (Anonymous 2005). Alternatives to traditional fishery-dependent stock assessment methods are thus needed (Cortés 1998). Demographic fishery-independent based analyses are particularly appropriate for the blue shark 17 since it has one of the most complete life-history datasets available for sharks (see synopsis by Nakano and Seki 2003 for blue sharks, worldwide). Demographic parameters for the unfished blue shark population are available and are reported in meta-analyses (Smith et al. 1998; Cortés 2002a; Frisk et al. 2004), one comparative analysis of pelagic sharks (Cortés 2007), and the gray literature (Heessen 2003; Campana et al. 2005; Takeuchi et al. 2005). While all the analyses indicate that P. glauca is a highly productive shark species, knowledge of responses to perturbations from different fishery scenarios is missing. An age-structured matrix population model was constructed to investigate blue shark demography and population dynamics. A stochastic framework that accounts for the uncertainty in the vital rates was implemented following Cortés (2002a). The demography of the population in unfished conditions was analyzed, and elasticity analysis was used to identify the vital rates (fecundity and survival of pups, juveniles and two adult stages) that contribute most to the rate of population growth (Heppell et al. 1999). The impacts of a variety of harvesting scenarios on population growth rates were examined and harvest rates that allow for a stationary population were estimated. While demography, and elasticity analysis, in particular, could offer a “first-cut” knowledge basis for conservation and management (Heppell et al. 1999), risk assessment analysis provides a framework to quantify risks that account for the inherent variability of natural systems (Burgman et al. 1993). The risk (probability) of 18 a 50% decline of the population with respect to pre-exploited levels was evaluated under different fishery scenarios. 2.2. MATERIALS AND METHODS 2.2.1. Age-Structured Model An age-structured matrix population model (Caswell 2001) was used to investigate the demography of the blue shark in the North Atlantic (definition of mathematical notation is given in Table 2.1). The projection model Ν t+1 MHN t (2.1) was used, in which Nt is the vector with numbers at each age in year t. The matrix M is a Leslie population projection matrix f0 s 0 M0 0 0 f1 f2 0 0 0 s1 0 0 0 0 0 0 sx 1 fx 0 0, 0 0 (2.2) in which the sx element is the annual natural survivorship term for age x. The fx elements represent the age-specific per-capita fecundity rates. A birth-pulse population and a postbreeding census were assumed (Caswell 2001). Accordingly, the first age-class (age 0) is represented by the newborn pups and the fecundity (fx) terms include the probability that a pregnant female survives and delivers the pups at the 19 end of the year (fx=sxmx), in which mx is the average number of female pups per female). The mx terms were calculated as the product of the number of pups per female and the female sex ratio of the litters, which was then divided by the length of the reproductive cycle in years. Thus, this is a female population growth model. Fishery exploitation was modeled following Lefkovitch (1967) and Caswell (2001). Gallucci et al. (2006) investigated the reproductive potential lost associated with alterative exploitation strategies for sharks in the same way. The method incorporates the use of a diagonal matrix, the harvest (or exploitation) matrix H, in the projection model. The elements of the harvest matrix, hx, are the proportions of individuals of age x surviving harvest. The model assumes that harvesting occurs prior to natural mortality and reproduction. Essentially, the augmented projection matrix MH has the same form as the original Leslie matrix M but is composed of reduced terms for both fecundity and survival of the harvested age-classes. Knowledge about the compensatory mechanisms of density-dependent population growth for sharks is mainly limited to theoretical hypotheses (Walker 1998). Conceptualizing any hypothesis of regulation based on competition for resources in a space-limited habitat becomes nothing more than speculation for the highly migratory blue shark, with a vast distribution throughout the North Atlantic. The dynamics of density-dependent growth was thus omitted from this research. The underlying assumptions of the matrix model (2.1) are that the population will grow exponentially and reach a stable age distribution (hereafter referred to as sad) (Caswell 2001). 20 2.2.2. Uncertainty of Life-History Parameters The biology of the blue shark has received substantial scientific attention to date (see Nakano and Seki 2003 for synopsis), but uncertainty in critical life-history parameters still exists. Uncertainty was accounted for using a simulation approach as in Cortés (2002), where statistical distribution functions where defined for each lifehistory parameter, based on published records. Age and growth - The age and growth of the blue shark in the North Atlantic is addressed by several authors (Stevens 1975; Aires-da-Silva 1996; Henderson et al. 2001; Skomal and Natanson 2003). Parameter estimates from the latter study were used since they were estimated from most of the blue shark size-range (Figure 2.1a). A maximum age (longevity) estimate of 15 years was determined empirically for females based upon interpretation of vertebral banding patterns (Skomal and Natanson 2003). The authors also provide theoretical estimates of longevity (21 and 26 years). Twenty one years may be most realistic, and agrees closely with Stevens (1975) theoretical estimate of 20 years. The empirical (15 years) and theoretical (21 years) values were used as the bounds of a discrete uniform probability mass function (pmf) for maximum age (Figure 2.1b). Reproduction - Pratt (1979) reported full sexual maturity at 185 cm FL for female blue sharks which translates to an age-at-maturity of about 6 years using the juvenile growth curves of Stevens (1975), Aires-da-Silva (1996) and Henderson et al. 21 (2001). Skomal and Natanson (2003) suggest faster growth and an earlier age-at-maturity at five years of age. The growth curve inconsistencies were regarded as a form of uncertainly. Accordingly, a “knife-edge” age-at-maturity was allowed to vary uniformly between 5 years (Skomal and Natanson’s hypothesis) and 6 years (other growth studies) (Figure 2.1c). The length-at-age growth curve for mature females (Skomal and Natanson 2003) was used to obtain a fecundity-at-age curve (Figure 2.1d). A positive linear relationship between litter size and fork length of the pregnant females for the Atlantic Ocean (Mejuto and García-Cortés 2005) was used to convert mean lengths-at-age to mean fecundities-at-age. A mean value of 0.5 was used for the sex ratio of the litters (Castro and Mejuto 1995; Mejuto and García-Cortés, 2005; A. Aires-da-Silva, unpublished data from the Azores). The length of the blue shark reproductive cycle in any region of the globe is yet not fully understood. A conservative estimate of a 2-year cycle was taken (Pratt 1979). Survivorship - Natural mortality for the blue shark was quantified using a set of indirect techniques that rely on relationships between life-history parameters and natural mortality (Cortés 2002a; Simpfendorfer et al. 2005). Estimates of the instantaneous rate of natural mortality M (hence survivorship, s=e-M) were generated by six indirect methods: 1) Hoenig (1983); 2) Pauly (1980); 3) Chen and Watanabe (1989); 4) Peterson and Wroblewski (1984); 5) Jensen’s (1996) age-at-maturity method; and, 6) Jensen’s (1996) K growth coefficient method. The mathematical 22 equations for each method are in Appendix A. Blue shark pups are born with a mean size between 37-47 cm FL (Pratt 1979) and probably become highly vulnerable to natural sources of mortality during their first year of life (Cortés 2000). Rapid juvenile growth should decrease and balance a high rate of natural mortality (Branstetter 1990). Thus, two life-stages were assumed when defining the probability density functions (pdf) for natural survivorship: 0-1 years and 2+ year old sharks. The triangular pdf can be used to represent the uncertainty in life-history parameters that precedes stochastic demographic work (Cortés 2002a; Cortés 2007). This distribution is particularly convenient because it allows a lower and upper bound for the parameter, and the assignment of a most likely value between this range. The lowest and highest estimates of survival derived from the six methods were taken as the bounds, and the median value was assumed as the most likely value in the triangular distributions for the two stages (Figure 2.1e,f). Defining a pdf in terms of natural mortality (M) is an alternative approach. The mean and coefficient of variation (CV) obtained for M across methods were used as parameter estimates to define a lognormal distribution. A lognormal error structure for M ensures that the transformed estimates and resulting pdf of annual survivorship (s=e-M) vary between 0 and 1 (Figure 2.1e,f). 2.2.3. Stochastic Demographic Analysis Virgin population - The productivity of the blue shark population was first examined for the unfished (virgin) status. A set of demographic parameters was 23 generated (Caswell 2001). The dominant eigenvalue of the Leslie M matrix is the finite rate of population increase (λ) at sad. The population doubling time follows as 2 t 2 ln (2.3) Net reproductive rate (R0) was defined as the rate of growth of the population from one generation to the next (see Caswell 2001 for matrix algebra). Generation time was calculated as the time T required for the population to increase by a factor of R0. T was computed to satisfy T R0 , hence T ln R0 ln (2.4) Elasticity (proportional change) analysis was used to gain insight into the influence that changes in reproductive and survival rates can have on the population growth rate, λ (Heppell et al. 1999; Caswell 2001). Elasticities of the matrix elements (eij) were calculated from eij aij vi w j w, v , (2.5) in which aij are the elements of the Leslie matrix M, v is the reproductive value column vector with elements vi, w is the sad row vector with elements wj, and w, v is the scalar product between v and w. The life-span was aggregated into four stages for elasticity analysis: pups (0 years), juveniles (1-4 years) and two adult stages (5-9 24 years and a 10+ years group). Fecundity and survival elasticities for these age-groups were calculated by aggregating elasticities across elements of interest (elasticities add up to 1). A stochastic approach was used to mimic natural variability and quantify the uncertainty in the demographic parameters as in Cortés (2002a). The Monte Carlo simulations consisted of randomly drawing values of the life-history parameters from the statistical distributions described earlier (Figure 2.1). These were used as elements in the Leslie matrix model and to derive estimates of the demographic quantities of interest. Statistical distributions for the parameters were based on 10,000 simulations and the 2.5th and 97.5th percentiles were used as approximate 95% confidence intervals. All demographic and simulation analyses were coded in the R Language for Statistical Computing (R Development Core Team, 2006). The sensitivity of the demographic estimates to the choice of the statistical distributions for the life-history parameters is an important consideration since there is an element of subjectivity in the selection process (Cortés 2002a; Cortés 2007). This is especially the case for survivorship, commonly the least known life-history parameter for sharks (Simpfendorfer et al. 2005). The sensitivity of the demographic estimates to the choice among the triangular and lognormal techniques described early for deriving the pdf for survivorship (Figure 2.1c,d) was examined. Harvested population - The harvest matrix model approach (1) was used to investigate the growth response of the population, in terms of λ, in a fishery where the 25 controlling variables are the harvest rate (u) and the age-of-first-capture (tc). Variability in the vital rates was accounted for by the stochastic framework described earlier. The triangular pdf was chosen to define survivorship in the harvest analysis because the results from the sensitivity analysis that follows show similar estimates of λ for the two different statistical distributions of survivorship. The underlying assumptions of the harvest analysis were: selectivity is “knife-edge” at tc, and the fraction harvested annually (u) is applied equally to all selected ages. The “stationary harvest” (us) was defined as the annual harvest rate u resulting in a stationary population size (λ= 1). Three different scenarios were considered for tc. The first scenario consisted of a harvest including the pups (tc=0 years). The second hypothesis was that the pups are not selected into the fishery, but all remaining juvenile and adult ages are harvested (tc=1 year). A third scenario assumed that the whole juvenile segment (0-4 years) is protected and the fishery selects only the adult segment (5+ years) of the population (tc=5). Estimates of us were determined iteratively and statistical distributions were produced as described earlier for the demographic parameters. 2.2.4. Population Projections and Risk Analysis A risk assessment (Burgman et al. 1993) was conducted to quantify the probability of a long-term population decline to critical levels. The risk analysis requires that a critical value be chosen, below which the population is said to be in risk of overexploitation. Maximum sustainable yield (MSY) for some pelagic shark 26 species has been postulated to be at or above 50% of their virgin biomass (Cortés 2007). In particular, it is close to 50% for the blue shark and shifted further toward virgin biomass for two of the less fecund Alopias species and shortfin mako (Isurus oxyrinchus). Accordingly, the 50% seems a reasonable estimate of the critical value for precautionary management. The mean stable age distribution was taken as the initial condition to initiate the population projections for the risk analysis. The sad assumption, however, represents one of the major shortcomings of traditional demographic analysis because it assumes that survival and reproductive rates are constant over time (Cortés 1998). This static view is unrealistic and could result in misleading optimistic interpretations based on the use of the dominant eigenvalue λ for population projection work (Tuljapurkar 1990). The sad assumption and dependence upon the dominant eigenvalue theory where relaxed in the projection work and risk analysis (Burgman et al. 1993). Instead of assuming that vital rates are fixed over time, they were allowed to vary randomly from time step to time step. This corresponds to a time-variant Leslie matrix (Mt) in the projection model (2.1). Forward population projections were made over a 15-year period, which is the empirical longevity estimate for female blue sharks (Skomal and Natanson 2003). This process was repeated 10,000 times using Monte Carlo simulations to provide a sample of population trajectories from which to calculate the risk statistic. 27 The harvest scenarios modeled were different combinations of harvest rates focusing on juvenile (1-4 years) and adult (5+ years) blue sharks. Pups (0 years) were assumed not to be selected into the longline fishery due to small mean size (37-47 cm FL; Pratt, 1979). Five levels of harvest were considered (u=0, 0.1, 0.2, 0.3, 0.4). Harvest rates were assumed constant across the juvenile and adult age-classes to allow for a tractable number of harvesting combinations. A constant harvest policy over the projection period was assumed. The population depletion obtained under each harvest strategy at the end of the projection period is dependent upon the depletion status in the starting year. Three hypotheses were considered for the initial population size: a population still at its preexploited levels (N0), at 75% of N0, and at 50% of N0. 2.3. RESULTS 2.3.1. Survivorship Estimates of natural survivorship (s=e-M) produced for ages 0-1 years by the six indirect methods averaged 0.73 (median=0.74; Table 2.2). A minimum value of 0.53 was obtained using the technique of Chen and Watanabe (1989), which relies on the von Bertalanffy growth parameters. The Hoenig (1983) method, assuming a maximum age of 21 years, provided the maximum estimate (0.82) among all methods. Survivorship for ages 2+ years averaged 0.78 (median=0.81). The method of Pauly (1980) used the von Bertalanffy growth parameters and a mean water temperature value, and generated the minimum estimate of 0.62. A maximum 28 estimate of 0.91 was produced by the method of Peterson and Wroblewski (1984), based on weight-at-age data. 2.3.2. Demographic Analysis Estimates of the finite rate of population increase (λ) averaged 1.23 year-1 when the triangular pdf was assumed for survivorship (Figure 2a) and the distribution for the population doubling times (t2) averaged 3.08 years (Figure 2b). A mean growth rate of 8.33 per-generation was estimated for the net reproductive rate R0 (Figure 2c), with the estimate of mean generation time (T) being 9.39 (Figure 2d). The stable age distribution (sad) under virgin conditions showed a dominance of juveniles (0-4 years) (Figure 2e). This segment represented, in average terms, 91.2% of the population in numbers. Pups alone accounted for 41.3% of the total number of animals in the population. In contrast, the adult segment (age-classes 5+) accounted for a mean proportion of 8.8% of the population at a sad. Age-classes 10+ represented less than 1% of total population numbers. Elasticity analysis showed that juvenile survival is the dominant contributor to population growth (mean aggregated elasticity of 57.7% across ages 0-4; Figure 2f). Comparatively, adult survival (5+ years) contributed much less to λ (survival elasticity of 30.7% aggregated across mature ages of 5+ years). The proportional contribution of fecundity to population increase was quantified as a mean elasticity value of 11.5%. 29 The demographic and elasticity statistics were rather insensitive to the choice between defining the pdf of survivorship using the triangular or the lognormal distribution (Table 2.1, Figure 2.2). The lognormal distribution generally led to a wider range of values as a result of the larger dispersion represented in the lognormal pdf (Figures. 2.1e,f). Nevertheless, the measures of central tendency for the analysis based on the lognormal distribution were very similar to those produced by the analysis based on the triangular distribution. The mean growth response of the population in terms of λ when the harvest rate (u) and age-at-first-capture (tc) are the controlling variables was represented using isopleths (lines of equal λ; Figure 2.3a). Population declines (λ<1) were found to be associated with harvesting strategies selecting the juvenile age-classes (0-5 years). The distributions obtained for the stationary harvest (us) associated with fisheries focusing on different population segments (defined by different values of tc) are shown in Figure 2.3b. A mean stationary harvest of 0.19 (median=0.19, 95% CI: 0.07-0.26) was obtained for a fishery starting at age 0 (pups) and including all age classes. The estimates for us barely increased to a mean value of 0.21 (median=0.22; 95% CI: 0.07-0.29) when pups were protected and harvesting started at age 1. Higher stationary harvest rates were possible (mean us=0.42; median=0.43, 95% CI: 0.150.63) when the whole juvenile segment was protected (0-4 years) and the exploitation focused on the mature sharks only (tc set to the age-of-maturity, 5 years). 30 2.3.3. Risk Analysis The stochastic framework produced a sample of individual population trajectories under varying harvest scenarios, while accounting for the annual natural variability in the vital rates (Figure 2.4). In this analysis, the risk of depleting the population to levels below 50% of the pre-exploited levels after a 15-year period of constant harvest rate was evaluated. The risk statistic depends on three factors: the harvest rates for the juveniles, for adults, and the initial population size relative to the pre-exploited level (N0). The risks associated with different levels for the juveniles and adults (from 0 to 40%) are provided in Table 2.4. The corresponding risk curves for the three hypotheses about the initial population size (N0, 0.75N0, and 0.5N0) are shown in Figure 2.5. For example, if a 30% harvest rate is considered for both juveniles and adults, there is a risk of 100% of the population falling below 50% of N0 for all three hypotheses about the initial population size. If a more conservative strategy is considered, e.g., a 20% harvest rate applied to juveniles and adults, there is zero risk of the population falling below 50% of N0, if the initial population sizes were still at N0 or at 0.75N0. However, there is an approximately 25% risk if the initial population size was 50% of N0. It falls to the manager to determine what is an acceptable risk level. The domain of the risk curves (Figure 2.5) can be narrowed to include all strategies where the risk is less than some acceptable level, e.g., 25%. Take an extreme case for adult harvesting as an example. If the strategy is to allow for a harvest rate of 30% on the 31 adults, then a juvenile harvest rate below 10% is required if the risk is not to exceed the acceptable 25% level for all three hypotheses about initial population size. 2.4. DISCUSSION This research quantitatively confirms that that the North Atlantic blue shark stock is highly productive within the shark group. An annual rate of increase of 1.23 year-1 (mean λ) and mean doubling time of 3.1 years (t2) indicate a high capacity for growth, in the absence of harvesting. The values for λ (=er) calculated in this study are consistent with the range of estimates obtained for the blue shark in previous analyses in deterministic (1.03-1.28, Heessen, 2003; 1.43, Campana et al., 2005) and stochastic analysis: mean=1.40 (95% CI: 1.28-1.53; Cortés, 2002); mean=1.41 (90% CI: 1.25-1.63; Takeuchi et al., 2005); mean=1.25 (90% CI: 1.15-1.37; Cortés, 2007). This high capacity for population growth ranked P. glauca third in terms of λ in a meta-analysis involving 38 sharks species (Cortés 2002a). Another meta-analysis (Smith et al. 1998) ranked the blue shark seventh among 26 shark species when the estimates of intrinsic rebound potential (another demographic statistic of productivity) were compared. These data substantiate that the blue shark is one of the most productive sharks, especially among the pelagic species. Elasmobranchs are traditionally placed on the K side of the life-history continuum defined by r/K selection theory (Musick 1999; Stevens 1999). The group is usually characterized by slow growth rates, long life spans, late maturity, and production of limited offspring after long gestation periods (Branstetter 1990; Hoenig 32 and Gruber 1990). This low reproductive output is responsible for the vulnerability of elasmobranchs to harvest, as shown by many cases of overexploitation (Cortés 2004). Although certainly K-selected among fish in general, the blue shark seems to be shifted to the r- side of the life-history spectrum among elasmobranchs. According to the analysis of life-history patterns by Cortés (2000), this strategy consists of large production of small offspring that become highly vulnerable to natural mortality. Such high natural mortality is counterbalanced by one of the highest fecundities documented among sharks (mean litter size of 37 pups, Mejuto and García-Cortes, 2005; reaching up to 82 pups, Pratt, 1979) and by fast early growth rates (Branstetter 1990) that result in nearly doubling of pup size over the first year (Stevens 1975; Skomal and Natanson 2003). Expansion through the pelagic environment may also play a key role in the successful life-history strategy exhibited by blue sharks. The relatively poor nutrient supply available in these oceanic oligotrophic waters may be balanced by diminished competition with other predators in an environment where space should not be a limiting factor, as opposed to coastal waters. The cosmopolitan distribution of P. glauca throughout the globe, and the possibility that it may be the most abundant pelagic shark species (Nakano and Seki 2003), reflect the success of its life-history strategy. However, the high productivity of blue sharks could be misleading, and should not compromise the necessity to manage the current numbers lost to directed and incidental mortality in the North Atlantic, or in any other region of the globe 33 (Bonfil 1994; Clarke et al. 2006b). The question should still be asked whether there is any vulnerable aspect of the blue shark population that could put their inherent productivity at risk and lead to overexploitation. Elasticity analysis confirmed that survival of the juvenile segment (0-4 years) is the key factor behind productivity. This pattern is common for long-lived species, and sharks in particular (Heppell et al. 1999; Cortés 2002a; Cortés 2007). Population declines (λ<1) could result even if low (0.2-0.3) harvest rates are applied to juveniles. Protection of the juveniles may actually be the easiest management action to implement if a sustainable exploitation is desired. This could be attained by sizelimits implemented using regulations on fishing gear. An alternative or supplemental option is protection on the nursery grounds. Even though the complex sexual and lifestage segregation patterns of the blue shark over the entire North Atlantic are not yet fully understood, catch and tagging records suggest that the main nursery grounds are located in the eastern North Atlantic (Casey 1985; Castro and Mejuto 1995). Management and conservation of the blue shark would therefore benefit from longline selectivity studies and research focusing on a better delineation and mapping of the nursery grounds in the North Atlantic. Implementation of either management policy is handicapped by the fact that the blue shark is caught primarily as bycatch in commercial longline fisheries for swordfish and tuna (Heessen 2003; Anonymous 2005). Gear or spatial-temporal limitations on these fisheries would have therefore socio-economic implications. Nevertheless, implementation of a seasonal constraint on the longline fisheries 34 overlapping in time and space with the nursery areas appears to be a practical approach to protect juveniles and to simultaneously minimize the impacts on these fisheries. An example is given by the swordfish longline fishery in the waters of the Azores, eastern North Atlantic. Length-frequency data collected in the Azores during the spring suggest that this region is an important nursery ground where juvenile blue sharks congregate during this season, at least (Aires-da-Silva et al. 2008a). Higher catches of juvenile blue sharks are obtained in the spring, while the high catch season for the swordfish occurs in the autumn. These two fishing seasons are markedly out of phase with each other. The extension of this asynchronous pattern from the waters of the Azores to surrounding nursery areas in the eastern North Atlantic requires further investigation. Protection of nurseries falls within the context of large-scale marine reserves for shark conservation (Baum et al. 2003). The efficiency of open-ocean reserves, however, is dependent upon the migratory patterns of the juveniles in these regions. Seasonal latitudinal migrations have been suggested for blue sharks in the eastern North Atlantic, and these patterns seem to be strongly correlated with variations in water temperature (Stevens 1976; Queiroz et al. 2005). Thus, quantifying the movement patterns of the juveniles and their residency times in the nurseries becomes a critical step in the optimal design of any marine reserve strategy. The analysis of risk has become a common practice in fisheries stock assessment (Punt and Hilborn 1997). In this paper, risk analysis lies at the interface 35 between demographic analysis and stock assessment. In the absence of a reliable fishery-dependent dataset that could form the basis for a stock assessment, the results from the risk analysis offer supporting information for management and conservation. First, they help to identify a critical reference point which could guide precautionary management. In particular, a maximum annual harvest rate of 20% for both juveniles and adults could be considered (Figure 2.5). Harvest rates below this threshold carry low risks (<25%) of population declines to levels below half of the pre-exploited size (N0). These low risks are independent of three hypotheses for the initial population size at the start of the harvest policy (N0, 0.75N0, and 0.5N0). Increasing the harvest rate above the 20% threshold for either juvenile or either adults individually, or both, results in rapidly increasing risk. An evaluation of the future prospects of the stock could be obtained from the risk analysis if an estimate of the current harvest rate were available. Unfortunately, the limitations of the fishery-dependent data introduce uncertainty in the estimation of this parameter. Nevertheless, a reliable estimate for the harvest rate could well be obtained in the near future. For this purpose, the large body of mark-recapture theory (Seber 2002) could be used to capitalize on approximately four decades of extensive tagging data accumulated by several programs conducted on both sides of the North Atlantic (Stevens 1976; Kohler et al. 1998; Kohler et al. 2002; Fitzmaurice et al. 2005; Mejuto et al. 2005). Quantitative mark-recapture analyses efforts using these data have been recently initiated (Aires-da-Silva et al. 2005; Campana et al. 2006). 36 As in any population modeling analysis, the assumptions about the population are central to the validity of the results. The matrix modeling framework is flexible and permits the inclusion of different biological hypotheses. The absence of compensation in the current version of the model implies exponential population growth while it is possible that population growth rates may be depressed in the real world leading to more conservative demographic and risk predictions. Therefore, some form of compensation should be included in subsequent models. This is difficult, however, given the lack of empirical data on compensation for the sharks of the open ocean. An alternative to empirical data could follow from simulation analyses for a variety of hypothetical scenarios of harvest and compensation, such as density-dependent changes in growth, reproduction, and mortality. An example of a scenario can be found in the hypothesis that small size blue shark pups may be more vulnerable to natural mortality (Cortés 2000). Thus, the role of density-dependent changes in pup survival would be a relevant topic for a simulation study. The present demographic analyses confirm the importance of juvenile survival for population growth. Thus, future blue shark population dynamics and stock assessment models that are not age- or stage-based are of limited value. In addition, inclusion of spatial and movement components could better describe the complex spatial segregation and migratory patterns of the population by life-stage and sex, as well as different harvest scenarios (e.g., exploitation of juveniles in the nurseries). Finally, the urgency for advances in management of global blue shark populations is currently an important conservation topic (Clarke et al. 2006b). It 37 seems that the blue shark life-history parameters do not differ greatly over the world’s oceans (Tanaka et al. 1990; Nakano and Seki 2003). While this paper is concerned with the North Atlantic region, applications of the demographic and risk analysis results to blue sharks in other parts of the world appear to be reasonable and are straightforward to implement. 38 Table 2.1. Definition of mathematical notation. Nt M sx fx mx Η hx MH w wj v vi t2 R0 T eij aij w, v us tc N0 Vector of numbers at age in year t Projection (Leslie) matrix Annual natural survivorship at age x Fecundity at age x Mean number of female pups per female at age x Harvest survival matrix with diagonal elements 1 hx Fraction surviving harvest at age x Augmented projection matrix Dominant eigenvalue of MH (or M in the unexploited case). The annual rate of increase of the population at SAD. SAD vector for M , satisfying Μw w Element in column j of the SAD vector w Reproductive value vector for M , satisfying v T Μ v T Element in row i of the reproductive value vector v Population doubling time (in years) Net reproductive rate at SAD Mean generation time Elasticities of the elements i,j in the Leslie matrix ( M ) The elements i,j in the Leslie matrix ( M ) Scalar product between v and w Sustainable harvest, annual harvest rate (u) resulting in a stationary population ( 1 ) Age at first capture in the fishery Pre-exploited level of the population 39 Table 2.2. Estimates of natural survivorship produced by the following indirect methods: 1) Hoenig (1983); 2) Pauly (1980); 3) Chen and Watanabe (1989); 4) Peterson and Wroblewski (1984); and 5) Jensen’s (1996) age-at-maturity and K growth coefficient methods. Age (yr) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 0-1 years 2+ years Hoenig w = 15 w = 21 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 0.76 0.82 mean 0.73 0.78 median 0.74 0.81 Pauly C&W 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.62 0.53 0.65 0.71 0.75 0.78 0.80 0.82 0.83 0.83 0.84 0.85 0.85 0.86 0.86 0.86 0.86 0.86 0.86 0.87 0.86 0.86 0.86 min 0.53 0.62 max 0.82 0.91 P&W 0.73 0.79 0.83 0.85 0.86 0.87 0.88 0.88 0.89 0.89 0.90 0.90 0.90 0.90 0.90 0.91 0.91 0.91 0.91 0.91 0.91 0.91 Jensen t mat 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 0.72 K 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 0.81 Table 2.3. Descriptive statistics for the demographic parameters: population rate of increase (λ), doubling time (t2), net reproductive rate (R0), mean generation time (T). Statistics for fecundity and survival elasticities for pups, juveniles (1-4 years), adult state I (5-9 years) and adult stage II (10+ years) are also given. Elasticities Method λ Demographic statistics t2 R0 T Fecundity Adult I Adult II Pups Survival Juvenile Adult I Adult II Triangular pdf on s Mean Median Lower 95% CI Upper 95% CI 1.232 1.242 1.072 1.356 3.080 3.197 2.274 9.874 8.328 7.720 1.883 17.874 9.393 9.392 8.319 10.437 0.094 0.093 0.077 0.110 0.022 0.022 0.015 0.028 0.115 0.116 0.106 0.126 0.462 0.464 0.423 0.502 0.272 0.260 0.226 0.312 0.035 0.034 0.019 0.055 Lognormal pdf on M Mean Median Lower 95% CI Upper 95% CI 1.241 1.255 0.974 1.424 3.548 2.954 10.891 8.559 0.807 33.679 9.382 9.403 7.999 10.669 0.095 0.093 0.076 0.115 0.021 0.021 0.013 0.030 0.116 0.114 0.105 0.128 0.464 0.457 0.420 0.512 0.270 0.282 0.215 0.314 0.034 0.033 0.017 0.058 15.924 40 41 Table 2.4. Risk (probability) of obtaining a population decline to levels below 50% of the pre-exploited levels (N0). The management options are different harvest rates for juveniles (ujuv) and adults (uadult). Three different hypotheses were considered for the starting population size: a population at N0, at 0.75 N0 , and at 0.50 N0. The shaded cells highlight risk values above 25%. Management options h juv h adult No harvest No harvest 0.1 0.2 0.3 0.4 0.1 No harvest 0.1 0.2 0.3 0.4 0.2 No harvest 0.1 0.2 0.3 0.4 0.3 No harvest 0.1 0.2 0.3 0.4 0.4 No harvest 0.1 0.2 0.3 0.4 Hypotheses for starting pop. size (%N 0 ) N0 0.75N 0 0.5N 0 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.03 0.29 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.02 0.25 0.38 0.77 0.99 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.01 0.23 0.37 0.79 0.99 1.00 1.00 1.00 0.00 0.00 0.00 0.00 0.01 0.19 0.35 0.80 1.00 1.00 1.00 1.00 1.00 1.00 1.00 0.00 0.00 0.11 0.29 0.74 0.99 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 42 a) VBGFs Probability mass Fork length (cm) 300 200 Stevens (1975) Aires-da-Silva (1996) 100 Skomal and Natanson (2003) b) Longevity 0.2 0.1 Henderson et al. (2001 0 0.0 0 2 4 6 8 10 12 14 15 16 17 Age (years) 20 21 80 d) Fecundity at age c) Age at maturity 60 0.50 # of pups Probability mass 19 Age (years) 0.75 40 0.25 20 0.00 0 3 4 5 6 7 8 5 7 9 11 Age (years) 13 15 17 19 21 Age (years) 10 Probability density 10 Probability density 18 e) Survivorship (0-1 years) 8 triangular s lognormal M 6 4 2 0 f) Survivorship - 2+ years 8 triangular s lognormal M 6 4 2 0 0.2 0.3 0.4 0.5 0.6 0.7 Survivorship 0.8 0.9 1.0 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 Survivorship Figure 2.1. Uncertainty in the life-history parameters of the North Atlantic blue shark. a) von Bertalanffy growth functions (VBGFs) from different studies; b) probability mass function (pmf) for longevity; c) pmf for age-at-maturity; d) fecundity-at-age relationship; e) and f) are the probability density functions of natural survivorship (s) for 0-1 years and 2+ year old sharks. 43 b) a) Density triangular s lognormal M 0.0 0.4 0.8 1.2 1.6 Population growth rate year 2.0 0 2 1 4 6 8 10 12 Population doubling time (years) d) Density c) 0 10 20 30 40 0 2 Net reproductive rate 6 8 10 12 Generation time (years) 0.8 0.8 e) Stable age distribution (SAD) Proportion 4 f) Elasticies 0.6 0.6 Fecundity triangular s 0.4 lognormal M 0.2 Survival 0.4 0.2 0.0 0.0 0 1 2 3 4 5 6 7 8 9 10 Age-class (years) 11 12 13 14 15+ Pup Juv Ad1 Ad2 Pup Juv Ad1 Ad2 Age-group Figure 2.2. Statistical distributions of demographic parameters for an unfished blue shark population: a) finite rate of population increase, λ; b) doubling time, tx; c) net reproductive rate, R0; and mean generation time, T. e) the stable age distribution, sad; f) elasticities (of fecundity and survival) for aggregated age-groups (pups – 0 years; Juv - juveniles, 1-4 years; Ad1 – small-size adults, 5-9 years; Ad2 – large-size adults, 10+ years). 44 Age at first capture (years) 14 a) 12 10 8 b) 6 4 2 0 0.0 0.2 0.4 0.6 0.8 1.0 Annual harvest rate b) tc = 0 years Density tc = 1 year tc = 5 years 0.0 0.2 0.4 0.6 0.8 1.0 Stationary harvest rate Figure 2.3. a) Isoclines for the average response in population growth rate (λ) to different harvest rates (u) starting at different ages-at-first-capture (tc). The solid line indicates a stable population (λ=1). Values above and bellow this line represent population growth (λ>1) and decline (λ<1), respectively. b) Distributions derived for the stationary harvest rate (us) for fisheries beginning at an age-at-first-capture of 0 years (pups), 1 year (juveniles) and 5 year (adults). Poulation numbers 45 2 4 6 8 10 12 14 Year Figure 2.4. Example stochastic population projections. Fifty individual trajectories over a 15-year harvesting period are presented. The harvest rates used in this example are the same (u=0.3) for both juveniles and adults. 1.0 46 Nstart ujuv =0 0.5 N0 0.75N0 0.0 0.50N0 0.1 0.2 0.3 0.4 0.2 0.3 0.4 0.2 0.3 0.4 1.0 0.0 0.0 0.5 ujuv =0.1 0.1 1.0 0.0 0.5 0.0 Risk ujuv =0.2 0.1 1.0 0.0 0.0 0.5 ujuv =0.3 0.1 0.2 0.3 0.4 1.0 0.0 0.0 0.5 ujuv =0.4 0.0 0.1 0.2 0.3 0.4 Adult harvest rate, uadult Figure 2.5. Risk curves for depleting the population to levels below 50% of its pre-exploited population size (N0). The five plots represent different harvest strategies defined by three factors: harvest of juveniles (ujuv), and adults (uadult), and the initial population size fraction of N0. 47 CHAPTER 3 A HISTORICAL INDEX OF ABUNDANCE FOR THE BLUE SHARK IN THE WESTERN NORTH ATLANTIC Equation Chapter 3 Section 1 3.1. INTRODUCTION There is concern about the population status of top predatory fishes, the preferred target species for global fisheries, and the impact that reduced predator abundance could have on marine ecosystems (Pauly et al. 1998). One meta-analytical estimate indicated that the current biomass of large predatory fish (mainly tuna and billfish) is less than 10% of their historic (pristine) levels in the world’s oceans (Myers and Worm 2003). Similar alarming concerns were extended to shark populations (Baum et al. 2003; Baum and Myers 2004; Myers and Worm 2005; Shepherd and Myers 2005; Ward and Myers 2005). Elasmobranchs (sharks and rays) have a life-strategy that consists of slow growth rates during long life-spans, late ages-of-maturity, and the production of limited offspring after long gestation periods (Hoenig and Gruber 1990; Musick et al. 2000). Historically, sharks have been subject to a few directed fisheries but the dominant source of fishing mortality is bycatch in a wide variety of global fisheries employing almost every type of fishing gear used (Bonfil 1994; Walker 1998). The world’s unregulated shark fin trade is a major contemporary threat for sharks (Clarke et al. 2006a; Clarke et al. 2006b). The poor record of sustainable harvesting of shark populations is cause for concern, considering the many case studies of overfishing in targeted shark fisheries (Anderson 1990; Cortés 2004). 48 Unfortunately, incomplete historical information on shark fishery statistics and questions about the fate of discarded catches has resulted in the classification of stock assessment analysis for sharks as data limited (Bonfil 1994). The blue shark, Prionace glauca (Carcharhinidae), is among the most wideranging of large-open ocean predators, and it may be the most abundant of all pelagic sharks in the global oceans (McKenzie and Tibbo 1964; Draganik and Pelczarski 1984; Nakano and Seki 2003). Analyses of trade records indicate that blue shark fins dominate Hong Kong’s shark fin trade (Clarke et al. 2006a) and that their global exploitation rate may exceed maximum sustainable yield (Clarke et al. 2006b). The relative contribution of blue sharks from each ocean basin to the global fin market remains unknown. Little is known about the differentiation of blue shark stocks in the world’s oceans. In the North Atlantic, the hypothesis of a single stock for management and stock assessment purposes is substantiated by extensive tag-recapture records (Casey 1985; Kohler et al. 1998). These include frequent trans-oceanic basin migrations of blue sharks, and a very limited number of tag-recapture records crossing the equator. Blue shark catches in the North Atlantic have historically resulted primarily from bycatch in multi-national pelagic longline fisheries that target tuna and swordfish (Heessen 2003; Anonymous 2005). Since the initiation of these fisheries over half century ago, different exploitation patterns and target fishing practices have been employed. Japan first deployed pelagic longline gear in its tropical Atlantic tuna fishery in the mid 1950s (Nakano 1993; Bonfil 1994). In the 1960s, other nations 49 including Spain, the U.S., Canada and the Faroe Islands, utilized longline gear to target tuna, swordfish, and porbeable sharks, with blue sharks as bycatch. New exploitation patterns emerged during the last decade in response to reduced international swordfish quotas. In particular, effort has been diverted to target blue sharks for both meat (food) production (Mejuto et al. 2002; Aires-da-Silva et al. 2008a) and for the fin trade (Clarke et al. 2006a). The development of markets for shark meat has been particularly strong in Europe (Fleming and Papageorgiou 1996; Fordham 2006). The stock status of blue sharks in the North Atlantic is currently ambiguous. Baum et al. (2003) reported a general pattern of “collapse” for shark populations in the western North Atlantic, with blue sharks declining dramatically (by 60%) since the mid 1980s. The International Commission for the Conservation of Atlantic Tuna (ICCAT; Anonymous 2005) carried out a preliminary stock assessment and concluded that current exploitation levels are sustainable and that current biomass levels are above the level capable of producing maximum sustainable yield (MSY). Concern was expressed by ICCAT, however, about the limited quality of the fishery data available. The shortcomings include incomplete landings and fishing effort statistics, limited data on the status of discards (dead or alive), and on post-release survival. ICCAT also recommended better fishery-dependent (and -independent) information such as indices of abundance, to improve the quality of future assessments (Anonymous 2005). 50 Reliable indices of abundance play a key role in tuning analytical stock assessments (Maunder and Starr 2003; Maunder and Punt 2004). They are particularly critical for the North Atlantic blue shark because of the uncertainty about historical levels of bycatch mortality. Catch rate based standardized indices of abundance already exist for the blue shark in the western North Atlantic. These include analyses of U.S., Canadian and Japanese commercial fisheries data (Cramer 2000; Cortés 2002b; Baum et al. 2003; Brooks et al. 2005; Campana et al. 2006) and recreational fishery data (Babcock et al. 2000; Brown 2000; Skomal et al. 2005; Campana et al. 2006). A fishery-independent survey index is also available (Simpfendorfer et al. 2002). Unfortunately, the relative abundance trends reported in these studies often conflict, ranging from increasing, to stable and to decreasing trends and usually cover the period from the mid 1980s to the present. The focus of this research is on the development of an index of abundance for western North Atlantic blue sharks that covers nearly half-century of exploitation. The goal is to gain a perspective on the “missing baseline” (Baum and Myers 2004; Ward and Myers 2005), when stock conditions would have been close to pristine, in comparison to abundance in recent years after approximately five decades of exploitation. This is done by linking pelagic longline catch records from recent fishery observer programs (1978-2000) with historical catch and effort observations from fishery-independent longline surveys that were undertaken decadely from the late 1950s to the 1980s. The historical fishery-independent data were obtained from an extensive data recovery program focusing on the archives of U.S. and Canadian 51 fisheries agencies. Generalized linear model techniques (Maunder and Punt 2004) were used to develop a standardized catch rate index (1957-2000). 3.2. MATERIALS AND METHODS 3.2.1. Data Selection Pelagic longline fishery-independent (survey) and –dependent (observer) records were collected from several sources (Table 3.1). Data from historical longline survey cruises were coded from original cruise reports, field fishing logs maintained by scientific personnel, and grant reports. Set specific gear, deployment, retrieval, and species composition data was coded as consistently as possible with the more detailed and recent observer series. A detailed description of the different datasets is found in Appendix B. A blue shark data subset was chosen to focus the analysis on the geographical areas with the longest time series, the largest proportion of the total number of blue shark observations available, and a reduced number of sets that caught no blue sharks (“zero sets”). The geographical strata (with numerical codes and acronyms) are the U.S. National Marine Fisheries Service reporting areas for the pelagic longline fishery (Beerkircher et al. 2003), specifically the Mid Atlantic Bight (5 - MAB), Northeast Coastal (6 - NEC), and Northeast Distant (7 - NED) strata. The spatial extent of the analysis is bounded on the south by 35N latitude (Cape Hatteras, North Carolina U.S.), extends to northern Newfoundland, Canada (up to 55 N), bounded on the east by 45 W longitude, the general range limit of U.S. 52 vessels. Sets south of 35 N latitude, including areas off Florida, in the Gulf of Mexico and in the Caribbean, were excluded because they accounted for a small proportion of the total blue shark catch recorded in the data, and there were a very limited number of research cruise records south of 35 N available prior to 1978. Shallow sets in depths less than 50 m north of 35 N were also excluded since incursions of blue sharks into these inshore waters seem to be heterogeneous along the U.S. east coast (Casey 1982). The resulting selection of blue shark fishery-independent (survey) data consisted of a total of 1,365 longline sets conducted from 1957 to 1994 (Table 3.2a). These sets targeted tuna, swordfish and pelagic sharks, of which 1,057 (77.4%) caught blue sharks (“positive sets”). The fishery-dependent (observer) data selection consisted of tuna and swordfish sets by U.S. vessels (n=1,632; 1985-2000), and tuna sets by Japanese vessels (n=4,711; 1978-1988) (Table 3.2b). The proportion of blue shark positive sets was high for both observer series, 86.6% and 83.7%, respectively. The selected and pooled observer and historical survey data, hereafter referred to as the “reconciled data”, contain longline observations from fishing operations targeting different species: tuna, swordfish or pelagic sharks (Table 3.3). Except for the years of 1973 and 1974, the reconciled data provides continuous annual coverage from 1957 to 2000. Substantial temporal and spatial overlap exists between the longline observation time series for different target species (Table 3.3, Figure 3.1). 53 3.3.2. Catch-Effort Standardization The real changes in blue shark abundance represented in the reconciled data are confounded with changes over time in fishing practices for different target species (tuna, swordfish and sharks). “Catch-effort (or catch rate) standardization” consists of the process used to remove biases and produce a reliable index of abundance (Hinton and Maunder 2004; Maunder and Punt 2004). Standardization of blue shark catch and effort data used Generalized Linear Models (GLMs) (McCullagh and Nelder 1989; Dobson 1991). GLMs provide a single theoretical framework in which the assumptions of error normality and constancy of variance of traditional linear regression models are relaxed. The central assumption of a GLM is the existence of a linear relationship between some function of the expected value of the response variable and the explanatory variables: g ( i ) i (3.1) in which, g is a monotone, differentiable link function between the systematic and random components of the model; i is the expected value of the ith random response variable Yi (the random component). The right-hand side of Eq. (3.1) is the linear predictor i , a linear sum of the effects of the explanatory variables (the systematic component): 54 i xTi β (3.2) in which, xi is an n1 vector of explanatory variables (covariates and dummy variables for individual levels of factors); β an n1 vector of parameters. The explanatory variables and the different levels considered for the catch-effort standardization analysis are shown in Table 3.4. In addition to the set of main factors, two interactions terms (year:area and year:target) were also included in the GLMs. Two types of GLMs can be applied depending upon the choice made for the response variable. The alternative approaches are models for catch rate or count data. Both model types were investigated to derive an index of abundance for the blue shark. While the nominal blue shark catch rates were computed to implement the first class of models, counts (catch in numbers) were considered for the latter. The number of hooks deployed and set duration (longline soak times) varied by data source. Accordingly, fishing effort was quantified in terms of “hook hours”, the product of total number of hooks deployed (h) and the soak time (s) for each set (Simpfendorfer et al. 2002). The catch per unit effort (CPUE) was computed as follows: 55 CPUE c 1, 000 hs (3.3) in which, c is the total number of blue sharks caught in the longline set; h the total number of hooks deployed; s is the soak time duration of the longline, computed as the time interval between the start set and end haul times of the set. The catch-effort standardization process frequently encounters set records with no catch of the species being investigated (Maunder and Punt 2004). In the blue shark reconciled dataset, only 11.4% of the total number of sets are “zero sets”. However, the percentage of zero sets recorded over time for each individual data set, and among data sets, was variable. Two statistical approaches that explicitly account for the zero catches were investigated. The “delta approach” (Lo et al. 1992; Vignaux 1994), the most common, models the probability of a “positive” (non-zero) set and the mean catch rate of the positive sets separately. The probability of observing a blue shark positive set was modeled using a binomial GLM with the logit link (i ): i 1 i i g ( i ) ln (3.4) 56 Two error distributions were used to model the catch rate of the positive sets. First, a lognormal GLM was used in which the identity link i is: i i (3.5) A gamma GLM was also applied with the log link defined by: i g (i ) ln(i ) (3.6) The final index of abundance was produced by multiplying the year effect generated from the binomial and lognormal (or gamma) models. The second approach considered models for count (integer) data that explicitly account for the zeros. A standard Poisson error model is the first logical choice (Maunder and Punt 2004), since this is the first error assumption traditionally used to model rare event count data. However, the catch of blue sharks is certainly not a rare event, as indicated by the large proportion of positive sets observed in the various target species data sets. In addition, Ortiz and Arocha (2004) presented a comparative analysis of the performance of different models for standardizing catch rates of rare non-target species in a longline fishery, and the Poisson model performed poorly. The negative binomial offers a more flexible error-model for count data given that it assumes a quadratic relationship between the mean and variance. This error model is more flexible than assuming that the variance is equal to the mean as in the Poisson model, which makes it more vulnerable to overdispersion problems (Punt et al. 2000b). An extensive review of the theory of negative binomial regression models 57 can be found in Hilbe (2007). According to the canonical form derived directly from the negative binomial PDF, the mean of y, conditioned on and is: 1/ y 1/ 1 1 f y; , 1/ 1 1 y , 1 (3.7) in which is the negative binomial ancillary or overdispersion parameter. The canonical link is given by: i g (i ) ln(1/ i 1) (3.8) The traditional negative binomial regression model with the log link (Eq 3.4) was applied (NB-2; Hilber 2007). Effort data (in hook hours) were treated as an offset explanatory variable in the negative binomial GLMs (Maunder and Punt 2004). The precision of the relative abundance indices was quantified using a bootstrap approach (Vignaux 1994). The bootstrap resampling process consisted of randomly sampling with replacement from the longline datasets and repeating the GLM analysis for each sample draw (n=1,000). The 2.5th and 97.5th percentiles of the indices were used as their approximate 95% confidence intervals. All GLM and bootstrapping were coded in the R Language for Statistical Computing (R Development Core Team, 2006). The Akaike Information Criterion (AIC) (Burnham and Anderson 2002) was used to choose the most parsimonious GLM for each error model. AIC is computed based on the log-likelihood of the model (l) and number of parameters (p): AIC 2 ln l 2 p (3.9) 58 The model which provided the lowest AIC was taken as the most parsimonious for each of the error assumptions considered. A stepwise algorithm (the stepAIC function; Venables and Ripley 1997) was used to implement the model selection. AIC may perform poorly in the number of parameters (p) is low when compared to the sample size (n). When the ratio n/K is small (<40), a second-order variant of AIC, AICc, is recommended (Sugiura 1978). In case that n is large with respect to K, then AIC should perform well. In this study, the ratio n/K is equal to 100 (K for the higher dimensional model is 64; n is equal to 6,413). Therefore, AIC should perform well (Burnham and Anderson 2002). Diagnostic plots were used to evaluate the performance of the different error models selected, and to choose which of the error models (longnormal or gamma) best fitted the CPUE data of the blue shark positive sets (McCullagh and Nelder 1989; Maunder and Punt 2004; Ortiz and Arocha 2004). For the lognormal error models, quantile-quantile plots were inspected to check for departures from a lognormal error distribution. Diagnostic plots for all error distributions included plots of the standardized residuals against the fitted values to check for systematic departures from model assumptions. A check of the variance function was also made by plotting the absolute value of the standardized residuals against the fitted values. 59 3.3. RESULTS 3.3.1. Model Selection Table 3.5 presents the model selection summary statistics for the GLM fits to the blue shark reconciled data. The three types of data analyzed were the proportion of positive (non-zero) sets, the CPUE (catch rates) of the positive sets and the catch count data. The binomial GLM fit to the data on the proportion of positive sets explained the smallest fraction (9.3%) of the total deviance observed. With respect to the CPUE of the positive sets, the lognormal model explained 57% of the total deviance, whereas the gamma model explained 71.7%. The negative binomial model fit to the blue shark count data explained 54.7% of the total deviance observed. None of the interaction terms considered (year:area and year:target) were retained in the AIC based model selection. Model selection results for GLMs fit separately to each of the individual target species datasets (Appendix B, Table B.2). Diagnostic plots were used to ascertain which of the error models best fit the blue shark observations. Figure 3.2 provides diagnostic plots for the blue shark reconciled data. The quantile (q-q) plots of the ordered residuals were found to be consistent with the expected (null) linear pattern of a lognormal distributed error (Figure 3.2a). The diagnostic on systematic departures from a lognormal error assumption shows a distribution of residuals consistent with the expected pattern (a mean of zero and constant variance across a wide range of fitted values) (Figure 3.2b). In contrast, the gamma and negative binomial models show declining trends for residual means and a non-constant range (variance) of residuals across the observed 60 range of fitted values (Figures 2c,d). These patterns indicate possible overdispersion problems with the gamma and negative binomial models. The test on the performance of the variance function for the lognormal model best resembled the expected null pattern (Figure 3.2e) (an approximate stable trend in the absolute value of the residuals across fitted values). The gamma and negative binomial diagnostics indicate non-stable trends (Figures 2f,g). Diagnostic plots for the three error models fit to the individual target species datasets area also available (Appendix B, Figures B.1., B.2., B.3). In general, the residual patterns described above for the reconciled data were also identifiable in the model fits to the individual target datasets. 3.3.2. Catch-Effort Standardization Inspection of diagnostic plots supported the choice of a lognormal error assumption to describe the blue shark catch rate data. A delta-lognormal error model that accounts for both the zero catch observations and the CPUE of the positive sets data was chosen to derive an index of abundance for the blue shark. The delta-lognormal index (Figure 3.3c) was obtained from the product of the yearly effects from the binomial (Figure 3.3a) and the lognormal models (Figure 3.3b). The estimated proportion of non-zero sets was generally high (>60%) (Figure 3.3a). However, inter-annual variability is observed over years associated with different target fishing practices or research cruise series. In particular, the data in the early 1950-60s show larger proportions of zero sets. This supports the need to account 61 for the zero sets when modeling the blue shark catch rate data, the main objective of the delta-GLM approach. The nominal CPUE show a few years of unusually high catch rates (late 1960s and 1970s) (Figure 3.3b). This high variability was greatly reduced in the catch-effort standardization process. The precision of the delta-lognormal yearly estimates is generally high in the time series (Table 3.6). More specifically, the yearly coefficients of variation (CVs) ranged from 0.09 to 0.39 with a mean CV of 0.18. Precision was noticeable higher during the last two decades as a result of increased sample sizes from observer programs. The results suggest two distinct stanzas of catch rates during the exploitation history. The first stanza is the period of generally higher CPUE values prior to 1980 (mean relative CPUE of 1.2). A second stanza of apparently lower catch rate values (mean relative CPUE of 0.82) is apparent during the 1980s and 1990s. A comparison between these two average levels indicates an approximate 30% decline for blue shark catch rates in the western North Atlantic since the mid 1950s. The higher variability of the estimates during the first stanza could allow for an interpretation of a slighter greater or lesser decline. However, the results provide little support for any interpretation other than that there has not been a pronounced decline of CPUE levels over the exploitation history. Noticeably, the catch rates for 1971 and 1977 are higher and more variable when compared with adjacent years. Delta-lognormal, delta-gamma and negative binomial models are also available for all individual target species datasets before data reconciliation (Appendix B, Figures B.4., B.5., B.6). In general, the standardized CPUE for the 62 individual target segments of data showed high variability as well as trends that are not consistent with the life-history of shark species. These issues, however, were greatly reduced after data were pooled to form a long time series of longline observations in what was defined as the reconciled dataset. The mean effects of selected operational, seasonal and geographical factors on the blue shark catch rates are shown in Figure 3.4. The probability of catching a blue shark was much higher for those fishing operations that targeted pelagic sharks when compared with swordfish and tuna sets (Figure 3.4a). Tuna fishing strategies had the lowest mean effect on the blue shark catches. Other dominant effects on blue shark catches for selected operational factors included smaller set sizes (Figure 3.4b), high proportions of light sticks (Figure 3.4c) and shallow logline sets (Figure 3.4d). The spring quarter (April-June) had a noticeable higher effect on the blue shark catch rates (Figure 3.4e). Geographically, longline gear deployed in the Northeast Atlantic Distant area (NED, Figure 3.4f) and over the continental shelf shallow waters had high effects (Figure 3.4g). 3.4. DISCUSSION This study presents standardized catch rates for blue sharks caught in the western North Atlantic during the past four decades (1957-2000, Figure 3.3). The time series begins in the mid 1950s when Japan introduced pelagic longline gear into the tropical Atlantic tuna fisheries (Wise and Le Guen 1969; Nakano 1993; Bonfil 1994). The historical survey information presented here provides perspective on the 63 “missing baseline” (Pauly 1995; Myers and Worm 2003) for blue sharks in the western North Atlantic. This “baseline” would provide insight into the virgin population levels. The results support a conclusion that blue shark CPUE in the region has declined to no more than about 70% of the catch rate levels at a time immediately after the pristine population levels (mid 1950s). This evaluation assumes that fishery related exploitation of blue sharks was negligible prior to the expansion of longline fisheries in the late 1950s and 1960s. The interpretation of catch rates as an index of abundance must be considered carefully. Catch rate analyses described in several recent reports provide the basis for claims that many of the world’s populations of large predatory fishes, including sharks, are severely depleted and are currently being exploited at unsustainable rates (Baum et al. 2003; Myers and Worm 2003; Baum and Myers 2004; Myers and Worm 2005; Shepherd and Myers 2005; Ward and Myers 2005). These catch rate analyses generated almost immediate contrary responses by several fishery scientists (Walters 2003; Burgess et al. 2005a; Burgess et al. 2005b; Hampton et al. 2005; Hilborn 2006; Maunder et al. 2006; Polacheck 2006; Sibert et al. 2006). It is therefore critical that blue shark standardized CPUE series are evaluated in terms of their reliability as a potential index of abundance. The use of catch rates as an index of abundance has traditionally relied on the assumption that CPUE is proportional to abundance (Harley et al. 2001). It also assumes that the catchability (the q coefficient of proportionality) does not change over time, when in fact violations of this assumption can be caused by several factors 64 (see Maunder et al. 2006 for review). One of these factors is changes in target fishing practices over time. Unfortunately, the pooling of catch rate data from different target practices is commonly the only means to increase sample sizes, increase temporal and spatial coverage, and improve the information available for data-limited species, such as bycaught shark species (e.g., Baum et al. 2003; Campana et al. 2005; Ward and Myers 2005). The assumption of a time-invariant catchability was violated when longline data targeting different species (tuna, swordfish, and pelagic sharks) were pooled to build the historical time series of blue shark catch rates. The operational fishing practices (time variables) varied among different targeting practices. Deployment of tuna sets generally begins close to sunrise, within the first half of the day. Swordfish sets are generally deployed after sunset and before midnight. Starting time for pelagic shark effort was more variable. Gear dimensions (lengths of dropper and gangion lines) vary, such that different configurations are designed to exploit specific fishing depths, often relative to the depth of the thermocline or migrating forage species. These varying fishing strategies generally exploit differences in diel vertical distributions and feeding behavior between swordfish, tuna and sharks (Carey and Robison 1981; Carey and Scharold 1990). Generalized linear models (GLMs) were used to capture and remove the effect of the factors which bias CPUE as an index of abundance, particularly changes in catchability as a result of the different target practices (Maunder and Punt 2004; Maunder et al. 2006). The critical question is whether the GLM could successfully 65 account for the variability in the fishing practices for different target species that exists in the data available from both the research cruises and commercial trips. This is a pre-requisite if the standardized CPUE are to be interpreted as a reliable index of abundance for blue sharks in the western North Atlantic. The diagnostic plots suggest that the delta-lognormal error model is a reasonable choice to explain the blue shark catch rate data. This is consistent with the results of Ortiz and Arocha (2004) in which the same error model assumption performed best with longline bycatch data, compared to other error models. The linkage of the historical longline survey data with the more recent observer records seems to be the main factor contributing to the success of the blue shark catch-effort standardization. The temporal and spatial overlap between the different target species datasets provides enough signal and contrast about the main effects of various factors (operational, seasonal and geographical) on the blue catch rates (Table 3.3; Figure 3.1) to allow effective standardization. Longline sets that targeted pelagic sharks had a much stronger effect on the blue shark catch rates than tuna and swordfish sets (Figure 3.4a). Swordfish sets had significantly higher blue shark catch rates than tuna sets. The standard configuration for tuna gear consists of a longline catenary (curve) configured to reach deeper waters at sunrise when tuna are more vulnerable. Swordfish sets are traditionally deployed at shallower fishing depths at night after dusk to exploit the negative phototaxis behavior of the species (Carey and Robison 1981). Blue sharks appear to be more vulnerable to longline gear set at shallower fishing depths and during crepuscular 66 hours (sunrise and sunset) when they seem to actively feed (Carey and Scharold 1990). The GLM successfully accounted for the significant positive effect of light sticks on the blue shark CPUE (Figure 3.4c). Walsh and Kleiber (2001) found the same significant effect with a generalized additive model and tree analyses for the Hawaii-based commercial longline fishery. The spring quarter (April-June) has a significantly higher effect on blue shark catch rates than other seasons (Figure 3.4e), coincident with the northern and inshore migration of blue sharks along the U.S. shelf (Casey 1982). Geographically, the northeast distant region (NED) showed a dominant effect on the blue shark catch rates (Figure 3.4f). This region includes the Grand Banks and dynamic frontal zones where high blue shark catch rates have been reported in other analysis (Baum et al. 2003; Campana et al. 2006). Sets on shallow waters of the upper continental shelf had a strong effect on the blue shark catch rates (50-99 m; Figure 3.4g), which seems inconsistent with the oceanic habitat preferences of the blue shark (Nakano and Seki 2003). However, these shallow fishing grounds are where research cruise effort targeted blue sharks for tagging, particularly the fishery-independent surveys described by Simpfendorfer et al. (2002). These surveys frequently deployed a smaller number of hooks compared to commercial operations (J. J. Hoey, NOAA-NMFS, personal observations as scientific crew member of the R/V Geronimo, 1978-1981). These target fishing practices are also reflected by the dominant effect on blue shark catch rates for sets categorized in the smallest set size (< 99 hooks; Figure 3.4b). 67 Baum et al. (2003) report a general pattern of collapse for pelagic shark populations in the western North Atlantic from 1985 to 2000, and blue sharks in particular, were estimated to have declined by 60% since the mid-1980s. The present study suggests an approximate 30% decline in the catch rates since the mid 1950s. The conclusions of Baum et al (2003) were challenged (Burgess et al. 2005a; Burgess et al. 2005b) for several reasons, including their reliance on self-reported logbook data which tend to be subject to under- or non-reporting bias. Logbook data were not included in the present study to minimize such biases. All records in the reconciled time series were collected by scientific personnel or trained observers. A fishery-independent catch rate study of blue sharks in the western North Atlantic reported that male blue sharks declined by 80% between the mid-1980s and early 1990s (Simpfendorfer et al. 2002), while female catch rates seemed unchanged. Such a large decline in male relative abundance is difficult to reconcile with the 30% decline since the mid 1950s reported in the present study. As recommended by Simpfendorfer et al. (2002), the index presented herein relies on a number of datasets covering a broader spatial and temporal range for the blue shark and likely provides a more representative index of abundance. The R/V Geronimo’s data analyzed by Simpfendorfer et al. (2002) is one of several pelagic longline survey series investigated in the present study. The lower rate of CPUE decline reported for blue sharks in this study when compared to the previous estimates is not surprising. Life-history demographic studies show that the blue shark is one of the most productive shark species in the 68 world’s oceans (Smith et al. 1998; Cortés 2002a; Aires-da-Silva and Gallucci 2007). Stable patterns of blue shark CPUE have been documented for longline fisheries in the Atlantic (Nakano and Clarke 2005) and Pacific oceans (Nakano and Seki 2003). Preliminary stock assessments of blue shark stocks in the North and South Atlantic by ICCAT (Anonymous 2005) reported that the current biomass may not be far from virgin levels. Similar results were also reported for blue sharks in the North Pacific Ocean (Kleiber et al. 2001; Sibert et al. 2006). The CPUE stanzas identified seem correlated with the history of pelagic longline exploitation in the western North Atlantic. The first stanza is the period of higher CPUE values prior to 1980 (Figure 3.3c). In this period, blue sharks were taken as bycatch in the tuna and swordfish fisheries from Japan, U.S. and Canada in the western North Atlantic. Blue shark markets did not exist and there were economic incentives for the fleets to actively avoid areas with high blue shark catch rates. A mercury closure period for the U.S. swordfish fishery was in effect from 1970 to 1978 (Hoey et al. 1989; Gibson 1998). The higher catch rates for the years of 1971 and 1977 may be due to a combined effect of increased abundance and the smaller sample sizes during the closure period (Figure 3.3c). The second stanza of slightly lower catch rates is apparent in the 1980s and the 1990s. The 1980s coincides with the rapid expansion of the U.S. swordfish fishery and the coincident westward expansion of the Spanish swordfish fleet to fishing grounds in the vicinity of the Grand Banks, where fishing effort overlapped with the North American and Japanese fleets. 69 There is supporting evidence from tagging studies that blue sharks in the North Atlantic represent a single stock (Kohler et al. 2002). Robust conclusions about the North Atlantic stock as a whole cannot be drawn from this study since the index of abundance is based upon catch and effort observations from the western North Atlantic only. Similar data are needed from the eastern North Atlantic to cover the entire distribution of the stock and recent fisheries which target blue sharks for food (Mejuto et al. 2002; Aires-da-Silva et al. 2008a). A stock assessment model that estimates the dynamics of the population integrating different sources of data, including CPUE indices, is needed if a reliable evaluation of the stock status is desired (Maunder et al. 2006; Sibert et al. 2006). Finally, recovery of longline historical data should be continued on both sides of the North Atlantic to improve catch rate indices for blue sharks and pelagic sharks in general. The catch-effort standardization modeling could be improved if additional gear (operational) variables are obtained. In particular, the GLM standardization could be enhanced from more refined evaluations of gear changes over time (e.g., changes of monofilament to multifilament, different bait types) within target strategies. Combining the longline catch and effort data with satellite derived environmental data (e.g., sea surface temperature, frontal zone data), and climatology indices (e.g., the North Atlantic oscillation index) could also refine the abundance indices. Table 3.1. Fishery-independent (research cruise) and –dependent (observer) pelagic longline data sources used to derive the blue shark index of abundance. Data sources a) Research cruise programs BCF-WHOI (1957-1970) BSFW-SHML (1961-1970) NARR (1975-1996) NARR-NE (1971-1973) NARR-comm (1977-1990) CN-DFO (1961-2000) b) Observer programs SEFSC-PLOP (1992-2000) NEFSC-PLOP (1990-1995) Dom-opport. (1985-1988) US Obs- JPN (1978-1988) Description Cooperative cruises by the Bureau of Commercial Fisheries and the Woods Hole Oceanographic Institution, U.S. Bureau of Sport Fisheries and Wildlife, Sandy Hook Marine Laboratory, U.S. National Marine Fisheries Service, Narragansett Laboratory, U.S. Cooperative cruises between NMFS Narragansett Laboratory and other institutions in the northeast U.S. Opportunistic deployments of NMFS Narragansett laboratory scientists aboard volunteer commercial vessels, U.S. Department of Fisheries and Oceans, Canada Southeast Fisheries Science Center, Pelagic Longline Observer Program Northeast Fisheries Science Center, Pelagic Longline Observer Program Opportunistic deployments aboard commercial vessels U.S. observers deployed aboard Japanese vessels 70 Table 3.2. Summary statistics of (a) fishery-independent and (b) fishery-dependent data sources that could form the basis for the estimation of blue shark abundance indices. Data source a) Research cruise programs BCF-WHOI BSFW-SHML NARR NARR-NE NARR-comm. CN-DFO b) Observer programs US domestic SEFSC - PLOP NEFSC - PLOP Dom-opport. US foreign US Obs. - JPN Target species Years # Sets # Pos. sets % Pos. sets Tuna, swordfish Coastal & pelagic sharks, swordfish, and tuna. Pelagic sharks Swordfish, pelagic sharks Swordfish Swordfish , Tuna 1957-1970 1966-1970 1975-1994 1971-1972 1977-1990 1963-1993 1957-1994 305 78 590 15 41 336 1,365 202 57 426 15 38 319 1,057 66.2 73.1 72.2 100.0 92.7 94.9 77.4 Swordfish, Tuna Swordfish, Tuna Swordfish 1992-2000 1990-1999 1985 1985-2000 629 984 19 1,632 537 862 15 1,414 85.4 87.6 78.9 86.6 1978-1988 1957-2000 4,711 7,708 3,942 6,413 83.7 83.2 Tuna Totals 71 72 Table 3.3. Annual time series of pelagic longline sets for the different target species. The acronyms for the longline sets are: (TUN) tuna, (SWF) swordfish, (PLSHK) pelagic sharks, (TUN-JPN) Japanese tuna sets, (TUN-US) U.S. tuna sets, (SWO-US) U.S. swordfish sets. Research cruises SWF PLSHK Year TUN 1957 1958 1959 1960 1961 1962 1963 1964 1965 1966 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 35 25 27 21 13 4 23 37 14 2 1 Totals % 213 2.8 468 6.1 684 8.9 # BSH pos % BSH pos 126 2.0 431 6.7 500 7.8 9 4 22 20 42 44 46 37 20 26 2 25 55 38 10 29 7 8 21 14 Observer programs TUN-JPN TUN-US SWO-US 35 25 27 21 13 4 23 37 14 2 1 13 21 25 13 15 7 9 19 33 64 75 46 49 23 32 24 46 48 3 10 26 10 56 2 10 5 TUN Reconciled data SWF PLSHK 9 4 22 20 42 44 46 37 20 26 2 25 55 38 10 216 255 316 758 843 279 338 496 421 500 289 19 216 255 316 758 843 279 338 496 421 500 289 29 7 8 19 13 21 25 13 15 7 9 19 33 64 75 46 49 23 32 24 46 48 3 10 26 10 56 2 10 5 Totals 35 25 27 21 17 26 43 79 58 61 59 45 48 15 32 57 0 0 47 19 43 280 330 391 814 866 311 370 561 469 503 299 26 41 104 181 348 304 260 23 137 76 102 155 7 14 31 128 121 96 14 65 24 39 25 3 34 148 196 178 164 9 72 52 63 130 7 14 31 128 121 96 14 65 24 39 25 24 34 148 210 178 164 9 72 52 63 130 4,711 61.1 564 7.3 1,068 13.9 5,488 71.2 1,536 19.9 684 8.9 7,708 100.0 3,942 61.5 442 6.9 972 15.2 4,510 70.3 1,403 21.9 500 7.8 6,413 83.2 Table 3.4. Categorical variables (factors) assumed in the blue shark catch-effort standardization. Acronyms are given for each factor. The different factor levels considered in the modeling are identified. Factors Acronyms Factor levels Area Bottom depth Light stick percentage Rig depth Set size Soak time Start set time Target species Quarter Year area btmD ltPer rigD setSz soakT startSetT tar quarter yr 5 -MAB, 6 - NEC, 7 - NED 50-99, 100-199, 200-499, 500-499, > 1000 meters 0 (no sticks) 1-24%, 25-49%, 50-74%, 75-100% of hooks Shallow - <= 12 hooks bet. floats; Deep - > 12 hooks bt flts. (Myabe, 1992) < 100; 100-299; 300-499, 500-999, >= 1000 hooks/set 0-8 h, 8-16 h, > 16 hours Dawn (0-8 h); Mid-day (8-16 h), dusk (17-24 h) Tuna, Swordfish, Pelagic sharks Jan-Mar, Apr-Jun, Jul-sep, Oct-Dec. 1957-2000 73 74 Table 3.5. Summary statistics of the selected four error models that were fit to the blue shark reconciled data. The explanatory factors, and the proportion of the total deviance explained are presented for each model. The models were a binomial GLM for the proportion of non-zero sets, a lognormal and gamma model for the catch rates of the non-zero sets, and a negative binomial model for the blue shark counts. The full text for the acronyms of the explanatory factors are provided in Table 3.4. Selected models % of dev. Proportion of positve sets - binomial yr + tar + quarter + area + btmD + setSZ + ltPer + rigD 9.26 CPUE of positive sets - Lognormal: yr + tar + quarter + area + btmD + setSZ + ltPer + rigD 57.00 - Gamma: yr + tar + quarter + area + btmD + setSZ + ltPer + rigD 71.66 Negative binomial - fish count data yr + tar + quarter + area + btmD + setSZ + ltPer + rigD 54.72 75 Table 3.6. Delta-lognormal standardized CPUE for the blue shark. The coefficient of variation (CV) is given for each yearly estimate. Year 1957 1958 1959 1960 1961 1962 1963 1964 1965 1966 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 Std. CPUE 0.98 0.48 1.11 1.18 1.13 1.50 0.70 0.87 1.55 1.27 1.43 1.31 1.96 0.97 1.08 1.93 0.88 0.75 1.82 1.06 0.86 0.83 1.05 0.78 1.01 0.68 0.74 0.48 0.50 0.44 0.80 0.94 1.22 0.63 0.95 0.98 0.73 0.47 1.25 1.16 0.76 0.78 CV 0.17 0.16 0.25 0.38 0.35 0.27 0.25 0.17 0.17 0.23 0.21 0.21 0.22 0.32 0.23 0.21 0.19 0.29 0.20 0.11 0.11 0.09 0.09 0.09 0.10 0.10 0.10 0.09 0.10 0.12 0.39 0.17 0.11 0.10 0.09 0.10 0.10 0.30 0.13 0.15 0.13 0.12 76 Tuna - research cruises 80° 70° 60° 50° Tuna - U.S., observer 80° 40° 70° 60° 50° 40° 50° 50° 50° 50° 40° 40° 40° 40° 30° 30° 30° 30° 80° 70° 60° 50° 80° 40° Swordfish - research cruises 80° 70° 60° 50° 70° 60° 50° 40° Swordfish - U.S. observer 40° 80° 70° 60° 50° 40° 50° 50° 50° 50° 40° 40° 40° 40° 30° 30° 30° 30° 80° 70° 60° 50° 40° 80° 70° 60° 50° 40° Pelagic sharks - research cruises Tuna - Japan, observer 80° 70° 60° 50° 80° 40° 70° 60° 50° 40° 50° 50° 50° 50° 40° 40° 40° 40° 30° 30° 30° 30° 80° 70° 60° 50° 40° 80° 70° 60° 50° 40° Figure 3.1. Geographical locations of pelagic longline sets for each target species fishing operation. 77 Figure 3.2. Diagnostic check plots for the different error models fit to the blue shark longline reconciled data set: lognormal and gamma models fit to the CPUE data of the non-zero sets, and negative binomial model fit to the count data. Left column: qqplots; middle column: diagnostics on systematic departures from model assumptions; right column: diagnostic on the performance of the variance function. 78 % pos. sets 1.0 0.5 a) Binomial 0.0 Year CPUE of non-zero sets 4 b) Lognormal 2 0 Year Delta-lognormal 4 c) Delta-lognormal 2 0 1950 1960 1970 1980 1990 2000 Year Figure 3.3. Delta-lognormal model estimates. a) proportion of positive sets from the binomial model; b) nominal (◦) and standardized CPUE (•) from the lognormal model on the CPUE of the positive sets; c) delta-lognormal estimates obtained from the product of a) and b). The vertical bars are the 95% confidence intervals of the estimates. Loess line smoothers are shown to help visualizing the average trends. 79 Figure 3.4. Mean effects of selected explanatory factors on blue shark catch rates. a) target species, b) set size, c) percentage of light sticks, d) rig length which defines the gear depth, e) geographical area, f) quarter, g) sea bottom depth. The effects are derived from the lognormal model on the catch rates of the positive sets. The vertical bars are the 95% confidence intervals of the estimates. 80 CHAPTER 4 A SPATIALLY-STRUCTURED TAGGING MODEL TO ESTIMATE MOVEMENT AND FISHING MORTALITY RATES FOR THE BLUE SHARK IN THE NORTH ATLANTIC OCEAN Equation Chapter 4 Section 1 4.1. INTRODUCTION The blue shark, Prionace glauca (Carcharhinidae), is a highly migratory apex predator which seems to be the most wide-ranging and abundant of all pelagic sharks in the global oceans (Draganik and Pelczarski 1984; Nakano and Seki 2003). Blue sharks are subject to fishing mortality caused by a great variety of fishing gear in the world’s oceans, but they are mostly taken as bycatch on pelagic longlines targeting tuna and billfish (Bonfil 1994). The global shark fin trade is a major threat to shark populations worldwide, particularly blue sharks, the dominant species in the shark fin trade (Clarke et al. 2006a). As other stocks of large pelagic fishes have declined, blue sharks are no longer discarded and finned exclusively, but are gradually becoming a source of meat in some markets (Castro et al. 1999; Mejuto et al. 2002; Aires-daSilva et al. 2008a). Unfortunately, the evaluation of the status of shark stocks worldwide is handicapped by limitations on fishery-dependent data and questions about the fate of discarded catches, among other uncertainties (Bonfil 1994). Despite these serious limitations, there has been progress in the field of shark population modeling (Cortés 2004). For the blue shark, in particular, stock assessments have been produced by some of the world’s Regional Fisheries Management Organizations (RMFOs). North 81 Pacific blue sharks were found to be exploited at sustainable levels (Kleiber et al. 2001; Sibert et al. 2006). Similar results were found for the South and North Atlantic blue shark stocks (Anonymous 2005). The strong reliance of these assessments on limited fishery-dependent data, however, calls for alterative (or supplemental) fishery-independent approaches (Aires-da-Silva and Gallucci 2007). Recent progress in the field of fishery-independent methods was the statistical modeling of shark fin trade records to produce estimates of catch and an evaluation of the blue sharks stocks globally (Clarke et al. 2006b). Unlike the optimistic results from the traditional stock assessments based on limited fishery data, the analysis of fin trade records indicated that the global exploitation levels of blue sharks may exceed maximum sustainable yields (Clarke et al. 2006b). Narrowing down this global evaluation to the ocean-basin level has not been made yet. Alternative (or supplemental) approaches to traditional stock assessments applied to sharks are life-history based demographic methods (Cortés 1998; Cortés 2002a; Gallucci et al. 2006). Demographic and risk analyses applied to the management and conservation of the North Atlantic blue shark are available (Airesda-Silva and Gallucci 2007). The analyses provide a means to evaluate the risk of population declines for a wide range of exploitation scenarios. However, the acquisition of a direct estimate of the current blue shark exploitation rate is still needed to evaluate the status of the blue shark stock using a demographic analysis framework. 82 Fish tagging data has long been used to estimate population parameters critical for management, such as population size, movement, growth and mortality rates (Beverton and Holt 1957; Quinn and Deriso 1999; Pine et al. 2003). The North Atlantic Ocean is particularly data-rich with respect to the availability of tagging data for the blue shark. These data have been accumulated for over almost a half century from tagging experiments conducted on both the western and eastern sides of the North Atlantic Ocean (Kohler et al. 1998; Kohler et al., 2002 for the western side; Stevens 1975; Stevens, 1990; Fitzmaurice et al. 2005; Mejuto et al. 2005, for the eastern side). The tagging data were critical to improve the understanding of the biology and ecology of North Atlantic blue sharks (Kohler et al. 2002; Skomal and Natanson 2003). In particular, tag-recovery records provided strong evidence in support of the highly migratory behavior of blue sharks in the North Atlantic and the trans-boundary nature of their fishery exploitation. This contributed to the hypothesis of a single North Atlantic blue shark stock for stock assessment and management purposes (Casey 1985; Anonymous 2005). Despite this important scientific information, quantitative modeling of tagging data for blue sharks has only been initiated recently (Aires-da-Silva et al. 2005; Campana et al. 2006). This study presents a tagging analysis using the blue shark tagging data of the National Marine Fisheries Cooperative Shark Tagging Program (NMFS-CSTP) to obtain estimates of movement and fishing mortality rates for the blue shark in the North Atlantic Ocean. Among all existing tagging programs, the CSPT presents the largest of the data sets and the broadest in terms of spatial coverage for blue sharks. It 83 is also the largest tagging data set available for any pelagic shark species worldwide. However, the program has relied mainly on opportunistic tagging by sport fisherman, which makes it difficult to implement any elements of experimental design. Future improvements could be made to obtain better quantitative population parameters, and these are discussed. 4.2. MATERIALS AND METHODS 4.2.1. Tagging Data Blue shark tagging in the North Atlantic Ocean has been carried out by the U.S. National Marine Fisheries Service Cooperative Shark Tagging Program (CSTP-NMFS) since the early 1960s. This tagging program is part of a continuous research project directed at the study of the biology of large Atlantic sharks (Kohler et al. 1998). An overview of the tagging methods and results obtained by the CSTP for pelagic sharks is available (Kohler et al. 1998). The tag recovery results for the blue shark were also described in detail (Casey 1985; Kohler et al. 2002; Kohler and Turner 2008). A subset of the blue shark tagging data covering the period from 1965 to 2004 was selected from the CSTP database for the tagging modeling. The data selection included only “M” dart tags (Kohler et al. 1998), which represent the great majority of the tags released on blue sharks. The blue shark data subset selected consisted of 95,301 tag release records and 5,859 tag recoveries over a large spatial extent throughout the North Atlantic (Figure 4.1). 84 4.2.2. Geographical Stratification and Definition of Fisheries A geographical stratification was made to account for the spatial distribution of the tag releases and recoveries (Kohler et al. 2002). Specifically, the North Atlantic Ocean, defined as north of the Equator, was divided into four geographical regions (Figure 4.1): Northwest (NW, west of 40oW and north of 25oN); Southwest (SW, west of 40oW and south of 25oN); Northeast (NE; east of 40oW and north of 25oN); and, Southeast (SE, east of 40oW and south of 25oN). The annual time series of blue shark tag releases and recoveries for each region are shown in Figure 4.2 (also see Appendix C, Table C.1 for sample sizes). In the North Atlantic, blue sharks are caught by commercial and sport fisheries (Heessen 2003). Bycatch in longline fisheries targeting tuna and swordfish from several nations is the predominant source of fishing mortality (Bonfil 1994; Heessen 2003). Fishing effort data are used to model the dynamics of the tag recoveries under exploitation by the different fisheries (Hilborn 1990). Total estimated longline fishing effort for the major longline fisheries (in total number of hooks by quarter of the year and 5x5º square) was made available by ICCAT (Anonymous 2007). Unfortunately, there is no fishing effort data available for the other commercial gears (e.g., purse seines) catching blue sharks. Since these contribute only to a minor source of the blue shark bycatch, this tagging analysis deals only with the major longline commercial fisheries, and the sport fisheries. The different fisheries catching blue sharks in the North Atlantic need to be defined. This definition was based upon the distribution of the tag recoveries by fisher 85 group in each of the geographical regions (Table 4.1), and the ICCAT fishing effort data. A fishery was defined as the exploitation by a fisher group (e.g., flag) in a particular region. Fisher groups with low numbers of tag recoveries were pooled to increase sample sizes (e.g. “Commercial-others”, and “Sport-others”). Fisheries with reported fishing effort data but no tag recoveries were also defined in the model (e.g, “Commercial-Venezuela” in the SE region). Table 4.2 presents a list of the 23 fisheries considered in this study. Tag-recovery maps for each fishery are show in Figures C.2 to C.11. Fishing effort data was not available for some of the longline fisheries. In such cases, constant fishing effort (set to 1) was assumed (Hilborn 1990). The annual time series of fishing effort and tag recoveries for the 23 fisheries are shown in Figure C.12. 4.2.3. Model Development The tagging modeling framework described below includes two sub-model components, a population dynamics model of tagged fish and an observation model, and a likelihood function is also needed to fit the model to the tag recovery data. These are described below. Population dynamics model of tagged blue sharks - The general method of Hilborn (1990) for analysis of fish movement from tag recovery data was modified to accommodate the blue shark tagging experiment and the multiple fisheries in the North Atlantic. The first component of the modeling framework is a population dynamics model which describes the survival and movement of each tagged group of 86 fish. In this study, a tag release group was defined as a group of blue sharks tagged in a geographical stratum during a particular year (131 blue shark tag release groups were modeled; Appendix C, Table C.1). The population dynamics model of Hilborn (1990) was rewritten to account for instantaneous natural mortality and tag shedding rates, similar to Xiao (1996): ( M F j ,t ) n f Nˆ i ,a ,t 1 Nˆ i , j ,t Ti , j ,t e p j ,a j 1 f (4.1) in which, Nˆ i ,a ,t = expected number of tagged fish of tag group i present in area a at time t; Ti , j ,t = number of tags released at the start of the year (“recruitment” of tags occurring once at the time of release) from tag group i in area j at time t; Fj f,t = instantaneous fishing mortality rate by fishery f in area j at time t; M = constant instantaneous natural mortality rate; = constant instantaneous long-term tag shedding rate (Type 2 in traditional terminology, Beverton and Holt, 1957); p j ,a = probability of movement from area j to a; The instantaneous natural mortality rate (M) was fixed at 0.22 yr-1, an estimate which was derived from a diverse set of empirical methods applied to the North Atlantic blue shark (Chapter 2; Aires-da-Silva and Gallucci 2007) . Direct estimates 87 of the shedding rate ( ) of the M-darts released by the CSTP have not been estimated. As described below, sensitivity analyses were conducted to assess the impact of different values for this parameter. Observation model - The second component of the modeling framework is an observation model which predicts the expected numbers of tag recoveries, which are then compared to the actual numbers of tags that are captured and reported by each fishery. The observation model is a Baranov catch equation (Beverton and Holt 1957): Ft f f f ˆ ˆ Ri ,t N i ,a ,t f M Ft f M Ft f f 1 e (4.2) in which Rˆi ,ft = expected number of tag recoveries from tag group i at time t by fishery f; and f = constant annual tag reporting rate of fishery f. The available blue shark size data is not adequate to model the selectivity patterns of the different fisheries. A simplistic assumption that the blue shark size ranges caught by the different fisheries are fully selected to the fishing gear was made. The instantaneous fishing mortality rate of each fishery f at time t, Ft f , was parameterized as follows: 88 Ft f q f Et f e t f (4.3) in which, qf = time-invariant catchability coefficient of fishery f , Et f = fishing effort (in numbers of hooks) of fishery f at time t, tf = annual deviates in the effort of fishery f at time t, assumed to be normally distributed, t N (0, 2t ) . The fishing effort data should not be considered to be known as reported, since its credibility is highly variable among fisheries. Therefore, Equation 4.3 assumes that there is process error in the relationship between the instantaneous fishing mortality (F) and fishing effort (E). The annual fishery-specific effort deviates ( t ) represent transient deviations in the effort-fishing mortality relationship (Fournier et al. 1998; Kleiber et al. 2006). Model fitting - The third component of the model is an objective function which is minimized to estimate the parameters of the population dynamics and observation models. The highly migratory nature of the blue shark and the large-scale of the fishery present various sources of variability in the tag recoveries. Some examples are the heterogeneous distributions of the various fleets in the North Atlantic, incomplete mixing of tags in this vast region, time-variant tag-loss and 89 reporting rates over time and space. The negative binomial likelihood is a logical choice to model tag-recovery data since it allows for substantial variation among observations (Cormack and Skalski 1992; Hampton and Fournier 2001; Stewart 2007). Assuming each tag release group to be independent, the full likelihood of the observed tag recoveries ( Ltags ) in log-space is: tags L = i a t f Rˆi ,fa ,t f log Ri ,a ,t log Ri ,a ,t 1 ( 1) Rˆ f R f log i ,a ,t i ,a ,t log( ) Ri ,fa ,t log( 1) ( 1) ( 1) (4.4) in which, = the standard gamma function, = is the parameter defining the degree of overdispersion, given as the ratio of the variance to the mean: = var( Ri.a ,t ) E( Ri ,a ,t ) . An important component of the total likelihood function is modeling of the annual fishing effort deviates as model parameters (Fournier et al. 1998). Ideally, these random effects would be integrated out analytically and estimates for the standard deviations of their distributions obtained. Unfortunately, this is very computationally intensive for non-linear models. An alternative approach is to use a penalized likelihood framework (i.e., to estimate the random effects as parameters in 90 the model). This is done in a similar way as modeling of recruitment deviations in catch-at-age models (Maunder and Deriso 2003). The annual effort deviations were assumed to be normally distributed so that t N (0, 2t ) . A penalty was added to the negative log-likelihood function to constrain the annual effort deviations which are unknown parameters to be estimated. Ignoring constants, the final objective function ( L ) for minimization becomes: L Ltags + f f2 t 2t 2f t (4.5) The penalized likelihood framework provides flexibility to account for the credibility of the fishing effort data by specifying different “weighting factors” of the tagging data from different fisheries. For this purpose, the fishery-specific standard deviations of the annual effort deviations ( 2 f ) are fixed to different values which t guide the specification of the different weights. A value of 0.4 or less indicates that the effort data is well determined, whereas a value of 0.5 or higher would represent a higher uncertainty and low reliability (Kleiber et al. 2006). Accordingly, the standard deviations of the effort residuals were fixed to one of two values: =0.4 if effort data was available; or =1 when effort data was not available and a proxy was taken (constant effort set at 1.0). Table 4.3 lists the “free” parameters which are estimated in the model. A total of 22 out of the 23 fishery-specific catchability parameters (q) were estimated since one fishery did not report any tags (Venezuela - SE). The catchability of this fishery 91 was shared with the estimated q of a similar fishery (Venezuela - SW). The tagging model requires that a hypothesis be made about the movement patterns of the blue shark in the North Atlantic. Based on a preliminary analysis of the tag recovery distributions over time, it was assumed that blue sharks can stay in the same region or move to the neighbor regions within the annual time step of the model (Figure 4.3). This hypothesis corresponds to a total of 12 movement parameters, of which 8 only are estimated in the model (the other 4 can be derived analytically as one minus the sum of other parameters for a given region). The remainder parameters in the model are the 599 annual fishing effort deviations ( ) and the data overdispersion parameter ( ) of the negative binomial distribution. A quasi-Newton minimization procedure was implemented (AD Model Builder©, Otter Research Ltd., 2000) to estimate the model parameters. 4.2.4. Model Hypotheses – Base Case Model and Sensitivity Analyses Assumptions also need to be made about the processes of tag shedding ( ) and tag reporting ( ). Unfortunately, direct estimates of these parameters are not available for the blue shark. “Best guess” estimates were defined based on expert opinion of NMFS-CSTP and ICCAT fishery scientists. Twelve scenarios which represent different hypotheses about the rates of tag shedding ( ) and reporting ( ) were investigated (Table 4.4). The base case model (scenario 6) assumed the “best guesses” for and , as described below. The remaining scenarios examine sensitivity analyses to alternative assumptions about and . 92 There are biological and operational reasons supporting the hypothesis that the blue shark tag shedding rates are low in the CSTP. Specifically, the blue shark skin is very thick along the dorsal musculature below the dorsal fin, particularly for females which bear severe tooth cuts caused by the aggressive mating behavior of males (Stevens 1974; Pratt 1979). The thick dermis apparently provides a strong anchor basis for the M-type tags after it is penetrated by the stainless still dart (N. E. Kohler, NMS-CSTP, personal field work observations). The dominant tagger group of volunteer sport fishermen in the CSTP is very experienced with blue shark tagging. For these reasons, a 10% ( =0.11 yr-1) value was taken as the “best guess” for the tag shedding rate. No shedding, 0% ( =0) and 20% ( =0.22 yr-1) were taken as alternative hypotheses for sensitivity analysis. Tag-induced mortality also needs to be considered when modeling tagging data (Beverton and Holt 1957). The monitoring of physiological data for blue sharks during angling time indicated that their resilience against tagging-induced stress is the highest among several large pelagic species (Skomal 2007). Also, observations of the rapid healing of tag-induced scars and of the good accommodation of M-tags in the blue shark musculature are common (N.E. Kohler, NMFS-CSTP, personal observations). For these reasons, tag-induced mortality was assumed to be negligible for blue sharks. Defining the scenarios for the tag reporting rates is more difficult due to the large number of fisheries in the model (23 fisheries in the tagging model). For tractability, different hypotheses about were made for the major fisheries only as 93 these are likely to impact the final results to the greatest extent. A “best guess” of 100% tag reporting ( =1) by the NW sport fishery (“NW-sport”) is a reasonable assumption considering the high cooperation between the U.S. sport fishery and the CSTP. For sensitivity analysis, an alternative hypothesis of =0.5 was defined for the this sport fishery. The commercial longline fisheries by Japan (“JPN-comm”) and Venezuela (“VEN-comm”) are major fisheries taking blue sharks. However, the low and even non-reporting of tags from regions of high fishing effort by these fisheries indicates that their reporting rates are likely to be low. Accordingly, =0.2 was defined as the “best guess” for “JPN-comm” and “VEN-comm”, and =0.5 as the alternative hypothesis for sensitivity analysis. Active tag reporting by Spanish fishery scientists indicates higher reporting of tag recoveries by the Spanish fisheries when compared with other commercial fisheries. A “best guess” of =0.5 was defined for “SPNcomm”. Alternatively, =0.2 was considered for sensitivity analysis. Non-reporting of tags by the remaining fisheries was assumed to be negligible ( =1). 4.3. RESULTS 4.3.1. Model Fit to Observed Tag Recoveries The base case model fit to the blue shark tag-recovery is shown in Figure 4.4. In general, the annual time series of tag recoveries estimated by the base case model were consistent with the observed tag recoveries in different fisheries. An estimated 94 value of 1.6 for the overdispersion parameter ( , ratio of the variance to the mean), indicates that the tag recovery data are slightly overdispersed. 4.3.2. Movement Parameters There are two types of movement parameters in the tagging model: the probability of staying in a region, and the probability of moving to a neighboring region. The movement parameter estimates and their CVs for the base case model are presented in Table 4.5a. In general, the annual proportions of blue sharks staying (residency rate) in a particular region exceeded the emigration rates by a factor of two, at least. The exception to this pattern was the southeast region (SE) for which the total annual emigration rate (57%) exceeded the residency rate (43%). However, the precision of the movement parameters in the SE was the lowest (CV range of 0.470.80), which could be due to the lower number of releases in that region (Figure 4.2). 4.3.3. Fishing Mortality Rates The fishing mortality (F) estimates derived from the base case model are shown for each region in Figure 4.5. Since the most recent years of the ICCAT’s fishing effort data are subject to updates, the F values for 2000 are chosen for comparative purposes (Table 4.5b). The critical reference point assumed for conservation, Fc=0.2 yr-1, is an estimate obtained from demographic and risk analysis for the North Atlantic blue shark stock (Chapter 2; Aires-da-Silva and Gallucci 2007). 95 The catchability estimates (q) derived for the different fisheries from the base case model are given in Table 4.6. Western North Atlantic (NW and SW regions) Total fishing mortality in the NW (Figure 4.5a) peaked in 2000 at a level of NW total 0.10 ( F2000 , CV=0.09), less than the 0.2 critical fishing mortality estimate (Fc). At the fishery-specific level in the NW, the fishing mortality levels for the sport fishery NWsport reached 0.028 in 2000 ( F2000 , CV=0.09). The estimates of F for the commercial UScomm NW CNcomm NW U.S. and Canadian fisheries were lower ( F2000 =0.003 yr-1; F2000 =0.001), but their precision levels were the lowest among all major fisheries in the NW (CV=0.29 and 0.55, respectively). The commercial longline fleets from Spain and SPNcomm NW Japan had the highest F estimates in the NW, F2000 =0.049 yr-1 (CV=0.13) and JPNcomm NW F2000 =0.018 yr-1 (CV=0.25), respectively. The F levels associated with the NW Spanish longline fishery increased since the mid 1990s. The total levels of blue shark fishing mortality in the SW region were the NW total lowest among the four regions ( F2000 =0.032 yr-1, CV=0.24; Figure 4.5b). The Venezuelan longline fishery contributed the most to fishing mortality in the SW VEN SW ( F2000 =0.013 yr-1, CV=0.41), followed by the Japanese and Spanish longline JPN SW SPN SW fisheries: F2000 =0.007 yr-1 (CV=0.47) and F2000 =0.004 yr-1 (CV=0.35), respectively. 96 Eastern North Atlantic (NE and SW regions) The blue shark fishing mortality rate estimates are heterogeneous across the North Atlantic (Figure 4.5). The exploitation rates in the eastern regions (NE and SE) exceeded by a factor of two, at least, the F estimates for the western regions (NW and SW). Total fishing mortality has gradually increased in the NE and SE regions to levels above 0.10 during the 1980s and 1990s. The total F estimates in the 1990s NE total exceeded Fc=0.20 ( F2000 =0.204 yr-1, CV=0.25). The Spanish longline fishery accounted for 55% of the blue shark total fishing SPN NE mortality in the NE in 2000 ( F2000 =0.113 yr-1, CV=0.29), followed by 18% and JPN NE 8%, by the longline fisheries of Japan ( F2000 PT NE ( F2000 =0.037 yr-1, CV=0.57) and Portugal =0.016 yr-1, CV=0.65). The remainder of the total F was taken by a pooled group of “other” minor commercial fisheries (14%) and a pooled sport fishery group (5%). In the SE region, the estimated total F was close to the Fc of 0.2 yr-1 in the late SE total 1990s ( F2000 =0.182 yr-1), but the true parameter value could be below or above the critical value (CV=0.42). The Japanese longline fishery accounted for the majority (approximately 40-60%) of the total blue shark fishing mortality over most of the study period, JPN SE F2000 =0.111 yr-1 (CV=0.53). The Spanish longline fishery accounted for 29% of the total F in the SE, SPN SE F2000 = 0.052. However, these interpretations are 97 subject to higher uncertainty considering the higher CVs (of about 0.5) of these two estimates. Sensitivity analyses Twelve different model scenarios were fit to the blue shark tag recovery data incorporating different hypothesis about the tag shedding rate ( ) and the tag reporting rates ( ) of the major fleets (see Table 4.4 for model definitions). The movement parameters showed high sensitivity to the different assumptions about and (see parameter estimates and CVs of sensitivities in Appendix C, Table C.2). Most noticeably, the parameter estimates showed higher sensitivity to the highest rate assumed for tag shedding ( =0.2). The blue shark fishing mortality regional estimates were generally robust to the different scenarios investigated for and (Figure 4.6). A minor exception occurred in the SE region in which noticeably higher estimates for the total F were obtained at the highest levels of tag shedding rates (20%). The F estimates and their CVs for the sensitivity analyses are given in Appendix C, Table C.3. 4.4. DISCUSSION Knowledge of the stock status of the blue shark in the North Atlantic remains uncertain due to the major limitations of the fishery statistics. The critical question that needs to be answered is “what are the current levels of blue shark fishing mortality (F) in the North Atlantic, and how do they relate to the reference points for 98 conservation?” At this point, the estimate of Fc=0.20 yr-1 for the overall North Atlantic blue shark stock obtained from demographic and risk analyses is useful for comparative purposes (Chapter 2; Aires-da-Silva and Gallucci, 2007). The tagging analysis indicated that the blue shark fishing mortality rates are heterogeneous across the North Atlantic (Figure 4.5). This result could be due to changes in the blue shark catchability across regions, and the different spatial distribution patterns of the various bycatch and target fisheries (Anonymous 2007). During approximately four decades of exploitation (1965-2000) in the western North Atlantic (NW and SW regions), the F estimates were consistently lower than 0.1yr-1, well below the Fc =0.20 yr-1 reference point for conservation. Low blue shark fishing mortality estimates (F 0.1 yr-1) for the NW region are consistent with the history of pelagic longline exploitation in this region. Specifically, blue sharks were taken as bycatch in the tuna and swordfish fisheries from the U.S., Canada, and Japan, and there were economic incentives for the fisheries to actively avoid areas with high blue shark catch rates. The westward expansion of the Spanish swordfish fleet to fishing grounds exploited by the North American and the Japanese fisheries occurred in the 1980s. In addition, the subsequent development in the 1990s of some European markets for blue shark meat stimulated new blue shark targeting practices by the Spanish fishery (Mejuto et al. 2002). These reasons could explain the recent increase in blue shark fishing mortality levels in the NW region (Figure 4.5). Nevertheless, the total blue shark fishing mortality levels in the NW have yet not exceeded the Fc =0.20 yr-1 reference point. This interpretation is supported by a 99 historical index of abundance which revealed a small decline ( 30%) of blue shark CPUE over approximately four decades of exploitation (1957-2000) in the NW region (Chapter 3; Aires-da-Silva et al., 2008b). The recent fishery-specific estimates of F obtained for the commercial longline fisheries by the U.S. and Canada in the NW (~0.003 and ~0.001, respectively) were much lower than those obtained for the sport fishery (~0.09). One explanation is that while blue sharks are caught as bycatch in the two commercial fisheries, they are actually the main target of the U.S. sport fishery. The estimated commercial fishing mortalities could also be negatively biased if the tag reporting rates assumed are too optimistic ( =1). In addition, the F values for the sport fishery are overestimates since a large proportion of the tagged blue sharks are caught by sport fisherman and returned alive to the waters without the tag. On the other hand, the F estimates for the U.S and Canadian commercial fisheries were estimated to be about 10% and 5% only of the F estimates for the Japanese and Spanish fisheries, respectively, which appears low. A non-mixing problem with the tags in the NW could also explain the low F estimates for the commercial fisheries. This issue is discussed further below. In the SW region, blue sharks were mainly caught as bycatch in the Venezuelan and Japanese pelagic tuna fisheries. The blue shark fishing mortality estimates for the SW were the lowest among all regions (Figure 4.5). This could be explained by the blue shark submerge into deeper, cooler waters in tropical and equatorial habitats (Casey 1982; Kohler et al. 2002). Therefore, the blue shark 100 catchability could be lower to the Venezuelan fishery mainly targeting yellowfin tuna, Thunnus albacares (Ortiz and Arocha 2004). On the other hand, the same behavioral pattern could also result in a higher catchability of the blue shark to the Japanese “deep longline gear” targeting bigeye tuna (Thunnus obsesus) in tropical waters. However, this is not confirmed by the q parameter estimates obtained for the Japanese fisheries in the NW and SW regions (Table 4.6). This could be due to regional differences in the operational fishing practices and the tag reporting rates by Japan. The majority of the fishing effort of the Japanese tropical tuna fishery in the Atlantic takes place in the SE and not in the SW region (Nakano 1993). Higher levels of fishing mortality (0.10 F 0.25) were estimated for the eastern North Atlantic (NE and SE regions; Figure 4.5). The NE region includes the traditional fishing grounds exploited by the Spanish longline fishery for swordfish, in which blue sharks are a dominant component of the bycatch (Mejuto et al. 2002). To a lesser extent, blue sharks were also subject to bycatch in the swordfish and tuna fisheries of Portugal and Japan. Reduced abundance of the targeted swordfish and new European markets for blue shark meat in the 1990s, resulted in a recent shift to blue sharks as a target species in the Iberian (Spain and Portugal) fisheries (Mejuto et al. 2002; Aires-da-Silva et al. 2008a). Emerging blue shark targeting practices could account for the higher F estimates in the NE in the last decade. The F estimates for the NE during the pre-1990 period, however, seem too high in comparison with later years, particularly for the Spanish fishery which was still developing and not targeting blue sharks yet. The F estimates could be refined when a better 101 understanding of potential catchability changes over time is available (e.g., swordfish vs blue shark target practices). In the SE region, blue sharks were also subject to bycatch or target fishing mortality in the Spanish longline fishery. However, the Japanese longline fishery was estimated to account for most of the blue shark fishing mortality in this southern region, which is the traditional fishing ground of the Japanese Atlantic tropical tuna fishery (Nakano 1993). The submergence of the blue shark into cooler deeper waters in the tropical and equatorial regions could explain higher levels of catchability of the blue shark to the Japanese deep-water longline gear targeting bigeye tuna in the SE (Table 4.6). The total levels of blue shark fishing mortality in the SE have reached the 0.2yr-1 reference point in the late 1990s, as similar to the NE. High levels of blue shark fishing mortality in the eastern waters of the North Atlantic have implications for management and conservation. While the NE region includes important blue shark nursery grounds where juvenile sharks are highly vulnerable (Mejuto and García-Cortés 2005; Aires-da-Silva et al. 2008a), the SE region seems to account for the highest aggregations of neonates and pregnant females in North Atlantic Ocean (Castro and Mejuto 1995; Mejuto and García-Cortés 2005). Demographic analysis showed the strong dependence of the blue shark population growth rate on the survival of the juveniles, and that population declines could result even if low (0.2yr-1 F 0.3 yr-1) fishing mortality rates are applied to juvenile (Chapter 2; Aires-da-Silva and Gallucci, 2007). Therefore, the emerging 102 targeting fisheries for blue sharks in the eastern North Atlantic (Mejuto et al. 2002; Aires-da-Silva et al. 2008a) should be monitored with caution. Blue shark migrations across thousands of miles of the North Atlantic Ocean are well known from the conventional tag recovery data (Casey 1985; Kohler et al. 2002). These migratory patterns were captured by the tagging model, as indicated by significant inter-regional movement rates. However, the model also indicated that the rates of movement across regions seem to be lower than the proportions of sharks staying in a region on a yearly basis. This suggests that a spatially-structured stock assessment is necessary to capture the stock dynamics. Lower rates of movement across regions represent an opportunity for conservation, as large-scale marine reserves could potentially be an efficient tool for management (Baum et al. 2003; Aires-da-Silva and Gallucci 2007). The large-scale of the blue shark biological scenario and the lack of an experimental design in the opportunistic tagging program, opens possibilities for violations of the underlying assumptions of tagging analysis. A major assumption of the tagging model is that the tagged and untagged fish are randomly mixed in the population (Brownie et al. 1985). Violations to this assumption are commonly referred to as the “nonmixing” problem (Hoenig et al. 1998; Latour et al. 2001). The CSTP seems particularly vulnerable to failures of the random mixing assumption since the majority of the tags are released opportunistically by U.S. sport fisherman in the western North Atlantic (Kohler et al. 1998). In addition to this unbalanced spatial design of the tag releases, the same sport fishery accounts for large numbers of tag 103 recoveries, working against a rapid dispersal of the tagged blue sharks. Therefore, the CSTP could benefit from an improved spatial design if population modeling work becomes a main goal. On the other hand, the highly migratory behavior of the blue shark seems to favor rapid dispersal of tags out of the U.S. sport fishing grounds. In late summer and autumn, blue sharks initiate southern movements as well as extensive offshore migrations (Casey 1982; Casey 1985; Kohler et al. 2002). In addition, the CSTP was able to expand its cooperative tagging efforts geographically, and substantial numbers of blue sharks have also been tagged and released in the waters of the eastern North Atlantic since the early 1970s (see Figure 4.2). Finally, the spatial stratification of the model decreased its vulnerability to the nonmixing problem. Incorrect assumptions about the processes of tag shedding and tag reporting could also result in major biases (Beverton and Holt 1957). The sensitivity analyses showed that the fishing mortality parameters were generally robust to the various assumptions made about the tag shedding rate ( ) and the tag reporting rates of the major fisheries ( ). In contrast, the movement parameter estimates were highly sensitive to the different assumptions made, particularly to higher levels of tag shedding ( =0.20). The true values of the movement parameters therefore remain uncertain. Therefore, it is critical that empirical estimates be obtained for the tag shedding and tag reporting rates. Double-tagging experiments will help to estimate the tag shedding rate (Beverton and Holt 1957). A high-reward tagging experiment seems an appropriate choice to estimate the fishery-specific tag reporting rates 104 (Pollock et al. 2001). The modern tools of satellite-based tracking (Block et al. 2005; Hulbert et al. 2005) could also be useful to validate the blue shark movement parameter estimates. An additional potential source of bias in this study is the assumption that the tag reporting rates for the different fisheries are constant over time. In the real world, changes of the tag reporting rates are likely to have occurred over the long historical periods of the fisheries. Simulation studies could be conducted to evaluate the impact of violations to the assumption of time-invariant tag reporting rates on the model results (Xiao 1996). It should be noted that the geographical strata assumed in the model were mainly defined based on the spatial distribution of the tag releases and recoveries (Kohler et al. 2002), which largely reflect the fishing effort patterns of the cooperative taggers, and the different fisheries catching blue sharks. Alternative geographical strata should be considered in the future, particularly region designations that account for biological (e.g., sex- and size segregation patterns of the stock) and oceanographic conditions. Geostatisical methods could be explored for this purpose (Isaaks and Srivastava 1989). The negative binomial likelihood was chosen to deal with the overdispersion of the tag-recovery data, an issue that is commonly encountered with tagging data of highly migratory species (Hampton and Fournier 2001). However, it is possible that additional overdispersion in the data still exists which results from violations to the model assumptions. One example is the assumption that the tags and the fishing effort 105 are randomly distributed in the four geographical strata considered, and this may not to be the case for some fisheries. Therefore, further corrections for overdispersion could potentially improve the parameter estimates (Hilbe 2007). This study indicates that there is potential in the tools of tagging analysis to improve the limited scientific information available for management and conservation of the North Atlantic blue shark. Although data-limited in terms of fishery statistics, this stock is the data-richest, in terms of tagging data, among the world’s stocks of highly migratory sharks. Unfortunately, the historical blue shark tagging programs in the North Atlantic (Stevens 1976; Kohler et al. 1998; Fitzmaurice et al. 2005; Mejuto et al. 2005), all suffer from unbalanced spatial designs as a result of their opportunistic nature. While the ideal requirements of experimental design are not implemented, the pooling of individual data sets across the ocean could greatly improve the quality of the model parameters. Increased sample sizes would allow for size- and sex- specific modeling, and to estimate size selectivity from the tagging data (Anganuzzi et al. 1994). Despite the high volume of tagging data available, the blue shark case study remains a data- and modeling-limited situation in terms of fishery statistics and even essential tagging parameters such as the rates of tag shedding and reporting. To compensate for these uncertainties, several assumptions had to be made in this analysis. One example is the assumption of annual fishery-specific effort deviates in the effort-fishing mortality relationship, as in MULTIFAN-CL (Fournier et al. 1998; Kleiber et al. 2006). This assumption was needed to account for varying levels of 106 credibility of the fishing effort data from the different fisheries. An important philosophical issue that should be noted is that there is a balance between data quality and model complexity. Therefore, improved quality and a better understanding of the longline fishing effort data could improve future blue shark tagging analyses. Finally, it remains to integrate the blue shark tagging model with a blue shark stock assessment model (Punt et al. 2000a; Hampton and Fournier 2001). The stock assessment uncertainties associated with the fishery statistics could be reduced from integrated tagging and population dynamics modeling (Maunder 2001). Furthermore, the integrated approach would provide a framework in which the unknown rates of tag shedding and reporting could be estimated. 107 Table 4.1. Blue shark tag recoveries reported by fishing fleet (nation or group) for each geographical region. The four geographical regions are: Northwest (NW), Southwest (SW), Northeast (NE) and Southeast (SE). (X) identifies a fishery for which there is reported fishing effort but no tag recoveries. Fisher group NW Geographic region SW NE SE Totals Sport Canada 13 US 3,008 North America 3,021 13 3,008 3,021 Commercial United States 510 Canada 213 Japan 228 Spain 1,005 Venezuela Portugal 22 1,978 19 7 35 84 145 5 300 34 339 13 117 X 130 529 213 253 1,457 84 56 2,592 Scientific cruises United States Commercial others Other Asian nations - commercial Other Caribean nations - commercial Other Eastern Atlantic nations - commercial Other South American nations -commercial Not reported Pooled Sport others Carribean - sport Eastern Atlantic - sport 69 1 24 3 5 11 67 70 1 9 4 3 6 40 73 20 1 10 13 143 1 9 41 79 25 Pooled 1 1 Totals 5,110 25 7 7 250 356 25 8 33 143 5,859 108 Table 4.2. List of the fisheries defined in the tagging model. Fishery Sport Region Tag recoveries US and Canada NW 3,021 Others pooled SW NE 25 7 US NW SW 510 19 Canada NW 213 Spain NW SW NE SE 1,005 35 300 117 Japan NW SW NE SE 228 7 5 13 Portugal NW NE 22 34 Venezuela SW SE 84 0 Scientific - US NW 69 Commercial Others pooled Totals NW SW NE SE Tag recoveries Fisheries 41 79 10 13 5,857 23 109 Table 4.3. Estimated parameters of the tagging model. Parameter type # of params. Fishery-specific catchabilities 22 Movement probabilities 8 Fishing effort deviations 599 Overdispersion 1 Total 630 Table 4.4. Model sensitivity analysis. Each model case defines a different hypothesis about the instantaneous tag shedding rate ( ) and the annual tag reporting rates ( ) of the major fleets. Model 1 2 3 4 Tag shedding rate, (year-1) 0 (0%) NW sport 1 1 1 0.5 Tag reporting rate, (%) Spain comm. Japan comm. 1 0.2 0.5 0.2 0.5 0.5 0.5 0.2 Venezuela comm. 0.2 0.2 0.5 0.2 5 6 - base case 7 8 0.11 (10%) 1 1 1 0.5 1 0.5 0.5 0.5 0.2 0.2 0.5 0.2 0.2 0.2 0.5 0.2 9 10 11 12 0.22 (20%) 1 1 1 0.5 1 0.5 0.5 0.5 0.2 0.2 0.5 0.2 0.2 0.2 0.5 0.2 11 0 111 Table 4.5. Movement and fishing mortality parameter estimates and their coefficient of variation (CV) obtained from the base case (model 6 in Table 4.4). Parameter Value CV a) Movement rates NW to NW to SW to NE 0.683 0.296 0.021 0.024 0.056 0.156 SW to SW to NW to SE 0.824 0.067 0.108 0.060 0.266 0.416 NE to NE to SE to NW 0.708 0.142 0.150 0.073 0.292 0.269 SE to SE to SW to NE 0.430 0.295 0.274 0.474 0.800 0.514 0.028 0.003 0.001 0.049 0.018 0.000 0.001 0.001 0.110 0.290 0.550 0.130 0.250 0.660 1.010 0.100 0.090 0.002 0.004 0.007 0.013 0.002 0.003 0.480 0.350 0.470 0.410 1.050 0.570 0.032 0.240 0.113 0.037 0.016 0.010 0.029 0.290 0.570 0.650 1.090 0.910 0.204 0.250 0.052 0.111 0.000 0.019 0.500 0.530 0.280 0.690 0.182 0.420 1.641 0.066 b) Fishing mortality (F) in 2000 Northwest (NW) NW sport U.S. comm.. Canada comm. Spain comm. Japan comm. Portugal comm. U.S. scientific Pooled comm. NW total F2000 Southwest (SW) U.S. comm. Spain comm. Japan comm. Venezuela comm. Pooled sport Pooled comm. SW total F2000 Northeast (NE) Spain comm. Japan comm. Portugal comm. Pooled sport Pooled comm. NE total F2000 Southeast (SE) Spain comm. Japan comm. Venezuela comm. Pooled comm. SE total F2000 c) Overdispersion - negative binomial 112 Table 4.6. Catchability (q) parameter estimates obtained for each fishery from the base case (model 6 in Table 4.4). Fishery Sport Region Catchability (q) US and Canada NW 0.0160 Others pooled SW NE 0.0016 0.0051 US NW SW 0.0023 0.0003 Canada NW 0.0017 Spain NW SW NE SE 0.0061 0.0038 0.0349 0.0024 Japan NW SW NE SE 0.0090 0.0082 0.0199 0.0278 Portugal NW NE 0.0004 0.0145 Venezuela SW SE 0.0132 0.0132 Scientific - US NW 0.0008 Others pooled NW SW NE SE 0.0010 0.0030 0.0160 0.0127 Commercial 113 a) NW NE SW SE b) Figure 4.1. Blue shark tagging data collected by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (1965-2004). (a) tag releases and b) tag recoveries. The four geographical regions defined in the tagging model are: Northwest (NW), Southwest (SW), Northeast (NE) and Southeast (SE). 1980 1990 5 0 1970 1980 year year SW Atlantic SE Atlantic 1990 2000 1990 2000 year 1990 2000 Recaptures 20 15 0 5 10 200 100 0 Releases 5 Recaptures 15 1980 0 10 1970 300 20 80 60 40 0 20 Releases Recaptures 10 15 20 25 100 0 2000 25 1970 50 Releases 0 2000 100 200 300 4000 Releases Recaptures Recaptures 400 150 NE Atlantic 0 Releases 6000 NW Atlantic 1970 1980 year 11 4 Figure 4.2. Annual time series of blue shark tag releases and recoveries in each region of the North Atlantic Ocean. 115 NW NE SW SE Figure 4.3. Movement hypothesis for the blue shark in the North Atlantic Ocean assumed in the tagging model. Blue sharks can stay in the same region or move to the neighboring regions within the annual time step of the model. The four geographical regions are: Northwest (NW), Southwest (SW), Northeast (NE) and Southeast (SE). 400 60 Sport-N. America (1) 200 NW 20 0 10 0 Tag recoveries by fishery 10 0 0 10 20 Com. - Portugal (15) 25 125 30 15 0 0 0 0 10 Com. - Spain (6) 5 10 20 Com. - Japan (11) 5 10 Com. - Venez. (17) 10 5 0 0 0 0 0 10 10 10 10 25 5 Com. - Portugal (16) 5 Sport - others (21) 5 0 0 0 0 0 10 20 10 Com. - Spain (8) 15 Com. - Japan (13) 5 0 0 1980 2000 1960 Com. - Venez. (18) 10 2000 1960 Com. - others (26) 5 0 1980 Com. - others (25) 5 30 Comm. - others (24) 5 0 Com. - Japan (12) 10 Sport - others (20) 50 1960 30 Com. - Japan (10) 5 Com. - Spain (7) SE 60 Com. - Spain (5) Com. - others (23) Com. - US (3) NE 250 Com. - Canada (4) 30 Survey - US (19) SW 50 Com. - US (2) 0 1980 2000 1960 1980 2000 Years 11 6 Figure 4.4. Base case model fit (solid lines) to the blue shark tag recovery observations (solid circles) for each fishery by geographical region. The four geographical regions are: Northwest (NW), Southwest (SW), Northeast (NE) and Southeast (SE). 117 0.5 0.4 NW 0.3 0.2 0.1 0.0 1970 1980 1990 2000 1970 1980 1990 2000 1970 1980 1990 2000 1970 1980 1990 2000 Fishing mortality rate by fishery (F) 0.5 0.4 SW 0.3 0.2 0.1 0.0 0.5 0.4 NE 0.3 0.2 0.1 0.0 0.5 0.4 SE 0.3 0.2 0.1 0.0 US & Canada - sport Portugal - comm US - comm Venezuela - comm Canada - comm US - surveys Spain - comm Pooled - sport Japan - comm Pooled - comm Years Figure 4.5. Fishery-specific instantaneous fishing mortality rates (F) in each geographical region as estimated from the base case model. The four geographical regions are: Northwest (NW), Southwest (SW), Northeast (NE) and Southeast (SE). A list of the different fisheries in each region is provided in Table 4.2. The dashed horizontal lines identify the reference point for conservation (Fc=0.20) 118 0% tag shedding 10% tag shedding 20% tag shedding 0.3 NW NW NW SW SW SW NE NE NE SE SE SE 0.2 0.1 0.0 0.3 Total fishing mortality rate (F) 0.2 0.1 0.0 0.3 0.2 0.1 0.0 0.3 0.2 0.1 0.0 1970 1980 1990 2000 1970 1980 1990 2000 1970 1980 1990 2000 NW-sport: 100%; SPN-comm: 100%; JPN-comm: 20%; VEN-comm: 20% NW-sport: 100%; SPN-comm: 50%; JPN-comm: 20%; VEN-comm: 20% NW-sport: 100%; SPN-comm: 50%; JPN-comm: 50%; VEN-comm: 50% NW-sport: 50%; SPN-comm: 50%; JPN-comm: 20%; VEN-comm: 20% Years Figure 4.6. Sensitivity analyses of the estimate of total instantaneous fishing mortality (F) to different scenarios about the tag shedding rate ( ) and the tag reporting rates ( ) of major fisheries. The different scenarios investigated are identified in Table 4.4 and in the bottom caption of the figure. The dashed horizontal lines identify the fishing mortality reference point assumed for conservation (Fc=0.2). 119 BIBLIOGRAPHY Aires-da-Silva A. (1996). Contribution to the knowledge of the age and growth of the blue shark, Prionace glauca (Carcharhinidae), in the North Atlantic. Undergraduate Thesis of the Marine Biology and Fisheries Licenciatura Degree. Universidade do Algarve, Portugal. Aires-da-Silva A., Pereira J. G. (1999). Catch rates for pelagic sharks taken by the Portuguese swordfish fishery in the waters around the Azores, 1993-1997. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 49, 300-305. Aires-da-Silva A., Taylor I., Punt A. E., Gallucci V. F., Kohler N. E., Turner P. A., Briggs R., Hoey J. J. (2005). A framework for estimating movement and fishing mortality rates of the blue shark, Prionace glauca, in the North Atlantic Ocean from tag-recapture data. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 58, 1073-1086. Aires-da-Silva A., Gallucci V. F. (2007). Demographic and risk analyses applied to management and conservation of the blue shark (Prionace glauca) in the North Atlantic Ocean. Marine and Freshwater Research 58, 570-580. Aires-da-Silva A., Ferreira R. L., Pereira J. G. (2008a). Blue shark catch rate patterns from the Portuguese longline fishery in the Azores. In 'Sharks of the Open Ocean'. (Eds M. Camhi, E. K. Pikitch and E. A. Babcock.) pp. 230-234. (Blackwell Publishing: Oxford.) Aires-da-Silva A. M., Hoey J. J., Gallucci V. F. (2008b). A historical index of abundace for the blue shark (Prionace glauca) in the western North Atlantic. Fisheries Research 92, 41-52. Anderson E. (1985). Analysis of various sources of pelagic shark catches in the Northwest and Western Central Atlantic Ocean and Gulf of Mexico with comments on catches of other large pelagics. In 'Shark catches from selected fisheries off the U.S. east coast'. (Eds E. D. Anderson, J. G. Casey, J. J. Hoey and W. W. N.) pp. 1-14. (U.S. Department of Commerce: Washington, DC.) 120 Anderson E. D. (1990). Fisheries models as applied to elasmobranch fisheries. In 'Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries'. (Eds H. L. Pratt, S. H. Gruber and T. Taniuchi.) pp. 473-484. (U.S. Department of Commerce: Washington, DC.) Anganuzzi A., Hilborn R., Skalski J. R. (1994). Estimation of size-selectivity and movement rates from mark-recovery data. Canadian Journal of Fisheries and Aquatic Sciences 51, 734-742. Anonymous (2005). Report of the 2004 inter-sessional meeting of the ICCAT subcommittee on by-catches: shark stock assessment. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 58, 799-890. Anonymous (2007). Report of the 2007 meeting of the sub-committee on ecosystems. International Commission for the Consevervation of Atlantic Tunas. Subcommittee on Ecosystems. Madrid, Spain - February 19-23, 2007. Babcock E. A., Pikitch E. K., McAllister M. K. (2000). Catch rates of blue shark (Prionace glauca) in the U.S. Atlantic recreational fishery. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 51, 1850-1858. Baum J. K., Myers R. A., Kehler D. G., Worm B., Harley S. J., Doherty P. A. (2003). Collapse and conservation of shark populations in the Northwest Atlantic. Science 299, 389-392. Baum J. K., Myers R. A. (2004). Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecology Letters 7, 135-145. Baum J. K., Kehler D., Myers R. A. (2005). Robust estimates of decline for pelagic shark populations in the northwest Atlantic and Gulf of Mexico. Fisheries 30, 27-30. Beerkircher L. R., Brown C. J., Lee D. W. (2003). Overview of the SEFSC pelagic observer program in the Northwest Atlantic from 1992-2000. International Commission for the Conservation of Atlantic Tunas, Collective volume of scientific papers 55, 331-349. 121 Beverton R. J. H., Holt S. J. (1957). 'On the Dynamics of Exploited Fish Populations.' (Chapman and Hall: London.) Block B. A., Teo S. L. H., Walli A., Boustany A., Stokesbury M. J. W., Farwell C. J., Weng K. C., Dewar H., Williams T. D. (2005). Electronic tagging and population structure of Atlantic bluefin tuna. Nature 434, 1121-1127. Bonfil R. (1994). Overview of world elasmobranch fisheries. FAO Fisheries Technical Paper 341. FAO, Rome. Branstetter S. (1990). Early life-history implications of selected carcharhinoid and lamnoid sharks of the northwest Atlantic. In 'Elasmobranchs as living resources: advances in the biology, ecology, systematics, and the status of the fisheries'. (Eds H. L. Pratt, S. H. Gruber and T. Taniuchi.) pp. 17–28. (U.S. Department of Commerce: Washington, DC.) Brooks E. N., Ortiz M., Beerkircher L. R., Apostolaki Y., Scott G. P. (2005). Standardized catch rates for blue shark and shortfin mako shark from the U.S. pelagic logbook and U.S. pelagic observer program, and U.S. weighout landings. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 58, 1054-1072. Brown C. A. (2000). Standardized catch rates of four shark species in the VirginiaMassachusetts (U.S.) rod and reel fishery. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 51, 1812-1821. Brownie C., Anderson D. R., Burnham K. P., S. R. D. (1985). 'Statistical inference from band recovery data - a handbook.' (United States Fish and Wildlife Service, Resource Publication 156: Washington, DC.) Burgess G. H., Beerkircher L. R., Cailliet G. M., Carlson J. K., Cortés E., Goldman K. J., Grubbs R. D., Musick J. A., Musyl M. K., Simpfendorfer C. A. (2005a). Is the collapse of shark populations in the Northwest Atlantic Ocean and Gulf of Mexico real? Fisheries 30, 19-26. Burgess G. H., Beerkircher L. R., Cailliet G. M., Carlson J. K., Cortés E., Goldman K. J., Grubbs R. D., Musick J. A., Musyl M. K., Simpfendorfer C. A. (2005b). Reply to "Robust estimates of decline for pelagic shark populations in the Northwest Atlantic and Gulf of Mexico". Fisheries 30, 30-31. 122 Burgman M. A., Ferson S., Akçakaya H. R. (1993). 'Risk Assessment in Conservation Biology. Population and Community Biology Series 12.' (Chapman & Hall: London.) Burnham K. P., Anderson D. R. (2002). 'Model Selection and Multimodel Inference: A Practical Information-theoretic Approach.' (Springer-Verlag: New York.) Cailliet G. M., Bedford D. W. (1983). The biology of three pelagic sharks from California waters, and their emerging fisheries: a review. CalCOFI Report 24, 57-69. Campana S., Marks L., Joyce W., Kohler N. (2005). Catch, by-catch and indices of population status of blue shark (Prionace glauca) in the Canadian Atlantic. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 58, 891-934. Campana S. E., Joyce W., Marks L., Natanson L. J., Kohler N. E., Jensen C. F., Mello J. J., Pratt H. L., Myklevoll S. (2002). Population dynamics of the porbeagle in the northwest Atlantic Ocean. North American Journal of Fisheries Management 22, 106-121. Campana S. E., Marks L., Joyce W., Kohler N. E. (2006). Effects of recreational and commercial fishing on blue sharks (Prionace glauca) in Atlantic Canada, with inferences on the North Atlantic population. Canadian Journal of Fisheries and Aquatic Sciences 63, 670-682. Carey F. G., Robison B. H. (1981). Daily patterns in the activities of swordfish, Xiphias gladius, observed by acoustic telemetry. Fishery Bulletin 79, 277-292. Carey F. G., Scharold J. V. (1990). Movements of blue sharks (Prionace glauca) in depth and course. Marine Biology 106, 329-342. Casey J. G., Mather F. J., Mason Jr. J. M., Hoenig J. (1978). Offshore fisheries of the middle Atlantic Bight. Marine Recreational Fisheries 3, 107-129. Casey J. G. (1982). Blue shark, Prionace glauca. Species synopsis. In 'Ecology of the Middle Atlantic Bight Fish and Shellfish, Monograph 15, Fish Distribution'. (Eds M. D. Grosslein and T. Azarovitz.) pp. 45-48. (MESA New York Bight Project, New York Sea Grant Institute: Albany, New York.) 123 Casey J. G. (1985). Transatlantic migrations of the blue shark: a case history of cooperative shark tagging. In 'Proceedings of the First World Angling Conference'. Cap d'Agde, France. (Ed. R. H. Stroud) pp. 253-268. (International Game Fish Association: Fort Lauderdale, Florida) Castro J. A., Mejuto J. (1995). Reproductive parameters of blue shark, Prionace glauca, and other sharks in the Gulf of Guinea. Marine and Freshwater Research 46, 967-973. Castro J. I., Woodley C. M., Brudek R. L. (1999). A preliminary evaluation of the status of shark species. Fisheries Technical Paper 380. FAO, Rome. Caswell H. (2001). 'Matrix population models. Construction, analysis, and interpretation.' (Sinauer Associates, Inc. Publishers: Sutherland, Massachusetts.) Chen S., Watanabe S. (1989). Age dependence of natural mortality coefficient in fish population dynamics. Nippon Suisan Gakkaishi 55, 205-208. Clarke S. C., Magnussen J. E., Abercrombie D. L., McAllister M. K., Shivji M. S. (2006a). Identification of shark species composition and proportion in the Hong Kong shark fin market based on molecular genetics and trade records. Conservation Biology 20, 201-211. Clarke S. C., McAllister M. K., Milner-Gulland E. J., Kirkwood G. P., Michielsens C. G. J., Agnew D. J., Pikitch E. K., Nakano H., Shivji M. S. (2006b). Global estimates of shark catches using trade records from commercial markets. Ecology Letters 9, 1115-1126. Compagno L. J. V. (1984). 'FAO species catalogue. Vol. 4. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Part 1 Hexanchiformes to Lamniformes.' (FAO: Rome.) Compagno L. J. V. (1999). Systematics and body form. In 'Sharks, Skates, and Rays: The Biology of Elasmobranch Fishes'. (Ed. W. C. Hamlett.) pp. 515. (The Johns Hopkins University Press: Baltimore.) Cormack R. M., Skalski J. R. (1992). Analysis of Coded Wire Tag Returns from Commercial Catches. Canadian Journal of Fisheries and Aquatic Sciences 49, 1816-1825. 124 Cortés E. (1998). Demographic analysis as an aid in shark stock assessment and management. Fisheries Research 39, 199-208. Cortés E. (2000). Life history patterns and correlations in sharks. Reviews in Fisheries Science 8, 299-344. Cortés E. (2002a). Incorporating uncertainty into demographic modeling: Application to shark populations and their conservation. Conservation Biology 16, 10481062. Cortés E. (2002b). Catches and catch rates of pelagic sharks from the northwestern Atlantic, Gulf of Mexico, and Caribbean. International Commission for the Conservation of Atlantic Tunas, Collective volume of scientific papers 54, 1164-1181. Cortés E. (2004). Life-history patterns, demography, and population dynamics. In 'Biology of Sharks and their Relatives'. (Eds J. C. Carrier, J. A. Musick and H. R. Heithaus.) pp. 449–470. (CCR Press: Boca Renton, Florida.) Cortés E. (2007). Comparative life-history and demography of pelagic sharks. In 'Sharks of the Open Ocean'. (Eds E. K. Pikitch and M. Camhi). (Blackwell Scientific: Oxford) Cramer J. (2000). Large pelagic logbook catch rates for sharks. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 51, 1842-1849. Dobson A. J. (1991). 'An Introduction to Generalized Linear Models.' (Chapman & Hall: London.) Draganik B., Pelczarski W. (1984). The occurrence of the blue shark, Prionace glauca (L.), in the North Atlantic. Reports of the Sea Fisheries Institute 19, 61-75. Fitzmaurice P., Green P., Kierse G., Kenny M., Clarke M. (2005). Stock discrimination of the blue shark, based on Irish tagging data. International Commission for the Conservation of Atlantic Tunas, Collective volume of scientific papers 58, 1171-1178. Fleming E. H., Papageorgiou P. A. (1996). 'Shark fisheries and trade in Europe.' (TRAFFIC Europe: Brussels.) 125 Fordham S. V. (2006). Shark alert: Revealing Europe's impact on shark populations. The Shark Alliance, Brussels. Fournier D. A., Hampton J., Sibert J. R. (1998). MULTIFAN-CL: a length-based, age-structured model for fisheries stock assessment, with application to South Pacific albacore, Thunnus alalunga. Canadian Journal of Fisheries and Aquatic Sciences 55, 2105-2116. Frisk M. G., Miller T. J., Dulvy N. K. (2004). Life histories and vulnerability to exploitation of elasmobranchs: Inferences from elasticity, perturbation and phylogenetic analyses. Journal of Northwest Atlantic Fishery Science 35, 2745. Gallucci V. F., Taylor I. G., Erzini K. (2006). Conservation and management of exploited shark populations based on reproductive value. Canadian Journal of Fisheries and Aquatic Sciences 63, 931-942. Garcés A. G., Rey J. C. (1983). Analisis de la pesqueria española de pez espada, Xiphias gladius, entre los años 1973 y 1981. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 18, 622-628. Garcés A. G., Rey J. C. (1988). La pesqueria española de pez espada (Xiphias gladius), 1973-1982. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 20, 419-427. Gibson C. D. (1998). 'The Broadbill Swordfishery of the Northwestern Atlantic: The Fishery's Economic and Natural History (early 1800s through 1995).' (Ensign Press: Camden, Maine.) Grey M., Blais A. M., Hunt B., Vincent A. C. J. (2006). The USA's international trade in fish leather, from a conservation perspective. Environmental Conservation 33, 100-108. Gubanov Y. P., Grigor'yev V. N. (1975). Observations on the distribution and biology of the blue shark Prionace glauca (Carcharhinidae) of the Indian Ocean. Journal of Icthyology 15, 37-43. 126 Hampton J., Fournier D. A. (2001). A spatially disaggregated, length-based, agestructured population model of yellowfin tuna (Thunnus albacares) in the western and central Pacific Ocean. Marine and Freshwater Research 52, 937963. Hampton J., Sibert J. R., Kleiber P., Maunder M. N., Harley S. J. (2005). Decline of Pacific tuna populations exaggerated? Nature 434, E1-E2. Harley S. J., Myers R. A., Dunn A. (2001). Is catch-per-unit-effort proportional to abundance? Canadian Journal of Fisheries and Aquatic Sciences 58, 17601772. Heessen H. J. L. (2003). Development of elasmobranch assessments DELASS. Final report of the DG Fish Study Contract 99/055. Netherlands Institute for Fisheries Research (RIVO), IJmuiden, The Netherlands. Henderson A. C., Flannery K., Dunne J. (2001). Observations on the biology and ecology of the blue shark in the North-east Atlantic. Journal of Fish Biology 58, 1347-1358. Heppell S. S., Crowder L. B., Menzel T. R. (1999). Life table analysis of long-lived marine species with implications for conservation and management. In 'Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals'. (Ed. J. A. Musick.) pp. 137-148. (American Fisheries Society: Bethesda, Maryland.) Hilbe J. M. (2007). 'Negative Binomial Regression.' (Cambridge University Press: Cambridge.) Hilborn R. (1990). Determination of fish movement patterns from tag recoveries using maximum-likelihood estimators. Canadian Journal of Fisheries and Aquatic Sciences 47, 635-643. Hilborn R. (2006). Faith-based fisheries. Fisheries 31, 554-555. Hinton M. G., Maunder M. N. (2004). Methods for standardizing CPUE and how to select among them. International Commission for the Conservation of Atlantic Tunas, Collective volume of scientific papers 56, 169-177. Hoenig J. M. (1983). Empirical use of longevity data to estimate mortality rates. Fishery Bulletin 81, 898-903. 127 Hoenig J. M., Gruber S. H. (1990). Life-history patterns in the elasmobranchs: implications for fisheries management. In 'Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries'. (Eds H. L. Pratt, S. H. Gruber and T. Taniuchi.) pp. 1–16 (U.S. Department of Commerce: Washington, DC.) Hoenig J. M., Barrowman N. J., Pollock K. H., Brooks E. N., Hearn W. S., Polacheck T. (1998). Models for tagging data that allow for incomplete mixing of newly tagged animals. Canadian Journal of Fisheries and Aquatic Sciences 55, 1477-1483. Hoey J., Mejuto J., Iglesias S., Conser R. (1988). A comparative study of the United States and Spanish longline fleets targeting swordfish in the Atlantic Ocean targeting swordfish North of 40ºN latitude. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 27, 230-239. Hoey J. J., Conser R. J., Bertolino A. R. (1989). The western North Atlantic swordfish. In 'Audubon Wildlife Report 1989/1990'. (Ed. W. Chandler.) pp. 457-477. (Academic Press: San Diego.) Hoff T. B., Musick J. A. (1990). Western North Atlantic shark fishery management problems and informational requirements. In 'Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries'. (Eds H. L. Pratt, S. H. Gruber and T. Taniuchi.) pp. 455-472. (U.S. Department of Commerce: Washington, DC.) Holden M. J. (1968). The rational exploitation of the Scottish-Norwegian stocks of spurdogs (Squalus acanthias L.). Fish. Investig. Ministry of Agriculture, Fish. Food., London, U.K. Holden M. J. (1973). Are long-term sustainable fisheries for elasmobranchs possible? Rapports et Proces-Verbaux des Reunions, Counseil International pour l'Exploracion de la Mer 164, 330-367. Holden M. J. (1974). Problems in the rational exploitation of elasmobranch populations and some suggested solutions. In 'Sea Fisheries Research'. (Ed. F. R. Harden Jones.) pp. 117-137. (Halsted Press, John Wiley & Sons Ltd.: New York.) 128 Holden M. J. (1977). Elasmobranchs. In 'Fish Population Dynamics'. (Ed. J. A. Gulland.) pp. 187-215. (John Wiley & Sons Ltd.: New Work.) Hulbert L. B., Aires-da-Silva A. M., Gallucci V. F., Rice J. S. (2005). Seasonal foraging movements and migratory patterns of female Lamna ditropis tagged in Prince William Sound, Alaska. Journal of Fish Biology 67, 490-509. Isaaks E. H., Srivastava R. M. (1989). 'An introduction to applied geostatistics.' (Oxford University Press) Jensen A. L. (1996). Beverton and Holt life history invariants result from optimal trade-off of reproduction and survival. Canadian Journal of Fisheries and Aquatic Sciences 53, 820-822. Kebe P., Restrepo V., Palma C. (2002). An overview of shark data collection by ICCAT. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 54, 1107-1122. Ketchen K. S. (1969). A review of the dogfish problem off the western coast of Canada. Journa of the Fisheries Research Board of Canada. MS Rep. Ser. 1048. Kleiber P., Takeuchi Y., Nakano H. (2001). Calculation of plausible maximum sustainable yield (MSY) for blue sharks (Prionace glauca) in the North Pacific. SWFSC Admin. Rep. H-01-02 and Dept. of Commerce. Kleiber P., Hampton J., Fournier D. A. (2006). MULTIFAN-CL User's Guide. 123 p. Kohler N. E., Casey J. G., Turner P. A. (1998). NMFS Cooperative Shark Tagging Program, 1962-93: An atlas of shark tag and recapture data. Marine Fisheries Review 60, 1-87. Kohler N. E., Turner P. A., Hoey J. J., Natanson L. J., Briggs R. (2002). Tag and recapture data for three pelagic shark species, blue shark (Prionace glauca), shortfin mako (Isurus oxyrinchus), and porbeagle (Lamna nasus) in the North Atlantic Ocean. International Commission for the Conservation of Atlantic Tunas, Collective volume of scientific papers 54, 1231-1260. 129 Kohler N. E., Turner P. A. (2008). The use of pelagic fish tagging data to determine stock structure with emphasis on the blue shark (Prionace glauca) in the North Atlantic Ocean. In 'Sharks of the Open Ocean'. (Eds M. Camhi, E. K. Pikitch and E. A. Babcock.). (Blackwell Publishing: Oxford.) Latour R. J., Hoenig J. M., Olney J. E., Pollock K. H. (2001). A simple test for nonmixing in multiyear tagging studies: Application to striped bass tagged in the Rappahannock River, Virginia. Transactions of the American Fisheries Society 130, 848-856. Lee D. W., Brown C. J., Jordan T. L. (1996). Overview of the SEFSC Pelagic Observer Program in the Northwest Atlantic from 1992-1994 (SCRS/95/103). International Commission for the Conservation of Atlantic Tunas, Collective volume of scientific papers 45, 355-365. Lefkovitch L. P. (1967). A theoretical evaluation of population growth after removing individuals from some age groups. Bulletin of Entomological Research 57, 437-445. Litvinov F. F. (2006). On the role of dense aggregations of males and juveniles in the functional structure of the range of the blue shark Prionace glauca. Journal of Icthyology 46, 613-624. Lo N. C. H., Jacobson L. D., Squire J. L. (1992). Indexes of relative abundance from fish spotter data based on delta-lognormal models. Canadian Journal of Fisheries and Aquatic Sciences 49, 2515-2526. Lopez A. M., McClellan D. B., Bertolino A. R., Lange M. D. (1979). Japanese Longline Fishery in the Gulf of Mexico, 1978. Marine Fisheries Review 41, 23-28. Maunder M. N. (2001). Integrated Tagging and Catch-At-Age Analysis (ITCAAN): Model Development and Simulation Testing. . In 'Spatial Processes and Management of Marine Populations'. (Eds G. H. Kruse, N. Bez, A. Booth, M. W. Dorn, S. Hills, R. N. Lipicus, D. Pelletier, C. Roy, S. J. Smith and D. Witherell.) pp. 123-146. (Alaska Sea Grant Program.) Maunder M. N., Deriso R. B. (2003). Estimation of recruitment in catch-at-age models. Canadian Journal of Fisheries and Aquatic Sciences 60, 1204-1216. 130 Maunder M. N., Starr P. J. (2003). Fitting fisheries models to standardised CPUE abundance indices. Fisheries Research 63, 43-50. Maunder M. N., Punt A. E. (2004). Standardizing catch and effort data: a review of recent approaches. Fisheries Research 70, 141-159. Maunder M. N., Sibert J. R., Fonteneau A., Hampton J., Kleiber P., Harley S. J. (2006). Interpreting catch per unit effort data to assess the status of individual stocks and communities. Ices Journal of Marine Science 63, 1373-1385. McCullagh P., Nelder J. A. (1989). 'Generalized Linear Models.' (Chapman& Hall: London.) McKenzie R. A., Tibbo S. N. (1964). A morphometric description of blue shark (Prionace glauca) from Canadian Atlantic waters. Journal of the Fisheries Research Board of Canada 21, 865-866. Mejuto J. (1985). Associated catches of sharks, Prionace glauca, Isurus oxyrinchus, and Lamna nasus, with NW and N Spanish swordfish fishery, in 1984. ICES CM 1985/H:42. Mejuto J., Iglesias S. (1988). Campaña comercial de prospeccion de abundancia de pez espada, Xiphias gladius L., y especies asociadas, en areas proximas a Grand Banks. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 27, 155-163. Mejuto J., García-Cortés B., de la Serna J. M. (2002). Preliminary scientific estimations of by-catches landed by the Spanish surface longline fleet in 1999 in the Atlantic Ocean and Mediterranean Sea. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 54, 1150-1163. Mejuto J., García-Cortés B. (2005). Reproductive and distribution parameters of the blue shark Prionace glauca, on the basis of on-board observations at sea in the Atlantic, India and Pacific Oceans. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 58, 951-973. 131 Mejuto J., García-Cortés B., Ramos-Cartelle A. (2005). Tagging-recapture activities of large pelagic sharks carried out by Spain or in collaboration with the tagging programs of other countries. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 58, 974-1000. Miyabe N. (1992). Trend in CPUE for swordfish caught by the Japanese longline fishery in the Atlantic Ocean. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 39, 484-496. Musick J. A. (1999). Ecology and conservation of long-lived marine animals. In 'Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals'. (Ed. J. A. Musick.) pp. 1-10. (American Fisheries Society: Bethesda, Maryland.) Musick J. A., Burgess G., Cailliet G., Camhi M., Fordham S. (2000). Management of sharks and their relatives (Elasmobranchii). Fisheries 25, 9-13. Myers R. A., Worm B. (2003). Rapid worldwide depletion of predatory fish communities. Nature 423, 280-283. Myers R. A., Worm B. (2005). Extinction, survival or recovery of large predatory fishes. Philosophical Transactions of the Royal Society B-Biological Sciences 360, 13-20. Nakano H. (1993). A review of the Japanese fishery and research on sharks in the Atlantic Ocean. International Commission for the Conservation of Atlantic Tunas, Collective volume of scientific papers 40, 409-412. Nakano H., Seki M. P. (2003). Synopsis of biological data on the blue shark, Prionace glauca Linnaeus. Bulletin of Fisheries Research Agency 6, 18-55. Nakano H., Clarke S. C. (2005). Standardized CPUE for blue sharks caught by the Japanese longline fishery in the Atlantic Ocean, 1971-2003. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 58, 1127-1134 132 Neves-dos-Santos M., Garcia A., Pereira J. G. (2002). A historical review of the bycatch from the Portuguese surface long-line swordfish fishery: observations on blue shark (Prionace glauca) and short-fin mako (Isurus oxyrinchus). International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 54, 1333-1340. Olsen A. M. (1959). Status of the school shark fishery in southeastern fishery in southeastern Australian waters. Australian Journal of Marine and Freshwater Research 10, 150-176. Ortiz M., Arocha F. (2004). Alternative error distribution models for standardization of catch rates of non-target species from a pelagic longline fishery: billfish species in the Venezuelan tuna longline fishery. Fisheries Research 70, 275297. Parker H. W., Stott F. C. (1965). Age, size and vertebral calcification in the basking shark, Cetorhinus maximus (Gunnerus). Zool. Meded. (Leiden) 40, 305–319. Pauly D. (1980). On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. Journal du Conseil International pour l'Exploration de la Mer 39, 175-192. Pauly D. (1995). Anecdotes and the shifting baseline syndrome of fisheries. Trends in Ecology & Evolution 10, 430-430. Pauly D., Christensen V., Dalsgaard J., Froese R., Torres F. (1998). Fishing down marine food webs. Science 279, 860-863. Peterson I., Wroblewski J. S. (1984). Mortality rate of fishes in the pelagic ecosystem. Canadian Journal of Fisheries and Aquatic Sciences 41, 11171120. Pine W. E., Pollock K. H., Hightower J. E., Kwak T. J., Rice J. A. (2003). A review of tagging methods for estimating fish population size and components of mortality. Fisheries 28, 10-23. Polacheck T. (2006). Tuna longline catch rates in the Indian Ocean: Did industrial fishing result in a 90% rapid decline in the abundance of large predatory species? Marine Policy 30, 470-482. 133 Pollock K. H., Hoenig J. M., Hearn W. S., Calingaert B. (2001). Tag reporting rate estimation: 1. An evaluation of the high-reward tagging method. North American Journal of Fisheries Management 21, 521-532. Pratt H. L. (1979). Reproduction in the blue shark, Prionace glauca. Fishery Bulletin 77, 445-470. Punt A. E., Hilborn R. (1997). Fisheries stock assessment and decision analysis: The Bayesian approach. Reviews in Fish Biology and Fisheries 7, 35-63. Punt A. E., Pribac F., Walker T. I., Taylor B. L., Prince J. D. (2000a). Stock assessment of school shark, Galeorhinus galeus, based on a spatially explicit population dynamics model. Marine and Freshwater Research 51, 205-220. Punt A. E., Walker T. I., Taylor B. L., Pribac F. (2000b). Standardization of catch and effort data in a spatially-structured shark fishery. Fisheries Research 45, 129145. Queiroz N., Lima F. P., Maia A., Ribeiro P. A., Correia J. P., Santos A. A. (2005). Movement of blue shark, Prionace glauca, in the north-east Atlantic based on mark-recapture data. Journal of the Marine Biological Association of the United Kingdom 85, 1107-1112. Quinn T. J., Deriso R. B. (1999). 'Quantitative fish dynamics.' (Oxford University Press: New York.) R (2006). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. Rey J. C., Mejuto J., Iglesias S. (1988). Evolucion historica y situacion actual de la pesqueria española de pez espada (Xiphias gladius). International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 27, 202-213. Ripley W. E. (1946). The soup-fin shark and the fishery. Fishery Bulletin 64, 7–37. Seber G. A. F. (2002). 'The estimation of animal abundance and related parameters.' (The Blackburn Press: Caldwell, New Jersey.) 134 Shepherd T. D., Myers R. A. (2005). Direct and indirect fishery effects on small coastal elasmobranchs in the northern Gulf of Mexico. Ecology Letters 8, 1095-1104. Sibert J., Hampton J., Kleiber P., Maunder M. (2006). Biomass, size, and trophic status of top predators in the Pacific Ocean. Science 314, 1773-1776. Simões P. R. (1999). By-catch of swordfish fishery in the Azores from 1987-96; an annotation on shortfin mako shark and blue shark. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 49, 283-287. Simpfendorfer C. A., Hueter R. E., Bergman U., Connett S. M. H. (2002). Results of a fishery-independent survey for pelagic sharks in the western North Atlantic, 1977-1994. Fisheries Research 55, 175-192. Simpfendorfer C. A., Bonfil R., Latour R. J. (2005). Mortality estimation. In 'Management Techniques for Elasmobranch Fisheries'. (Eds J. A. Musick and R. Bonfil.) pp. 165-185 (FAO: Rome.) Skomal G., Babcock E. A., Pikitch E. K. (2005). Indices of blue and mako shark abundance derived from U.S Atlantic recreational fishery data. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 58, 1034-1043. Skomal G. B., Natanson L. J. (2003). Age and growth of the blue shark (Prionace glauca) in the North Atlantic Ocean. Fishery Bulletin 101, 627-639. Skomal G. B. (2007). Evaluating the physiological and physical consequences of capture on post-release survivorship in large pelagic fishes. Fisheries Management and Ecology 14, 81-89. Smith S. E., Au D. W., Show C. (1998). Intrinsic rebound potentials of 26 species of Pacific sharks. Marine and Freshwater Research 49, 663-678. Stevens J. D. (1974). The Occurrence and Significance of Tooth Cuts on Blue Shark (Prionace glauca L) from British Waters. Journal of the Marine Biological Association of the United Kingdom 54, 373-378. 135 Stevens J. D. (1975). Vertebral rings as a means of age determination in blue shark (Prionace glauca L). Journal of the Marine Biological Association of the United Kingdom 55, 657-665. Stevens J. D. (1976). First results of shark tagging in the north-east Atlantic, 19721975. Journal of the Marine Biological Association of the United Kingdom 56, 929-937. Stevens J. D. (1990). Further Results from a Tagging Study of Pelagic Sharks in the North-East Atlantic. Journal of the Marine Biological Association of the United Kingdom 70, 707-720. Stevens J. D. (1999). Variable resilience to fishing pressure in two sharks: the significance of different ecological and life history parameters. In 'Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals'. (Ed. J. A. Musick.) pp. 11-15. (American Fisheries Society: Bethesda, Maryland.) Stevens J. D., Bonfil R., Dulvy N. K., Walker P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. Ices Journal of Marine Science 57, 476-494. Stewart I. J. (2007). Defining plausible migration rates based on historical tagging data: a Bayesian mark-recapture model applied to English sole (Parophrys vetulus). Fishery Bulletin 105, 470-484. Sugiura N. (1978). Further analysis of the data by Akaike's information criterion and the finite corrections. Communications in Statistics, Theory and Methods A7, 13-26. Takeuchi Y., Senba Y., Nakano H. (2005). Demographic analysis on Atlantic blue and shortfin mako sharks. International Commission for the Conservation of Atlantic Tunas, Collective Volume of Scientific Papers 54, 1231-1260. Tanaka S., Cailliet G. M., Yudin K. G. (1990). Differences in growth of the blue shark, Prionace glauca: technique or population? In 'Elasmobranchs as Living Resources: Advances in the Biology, Ecology, Systematics, and the Status of the Fisheries'. (Eds H. L. Pratt, S. H. Gruber and T. Taniuchi.) pp. 177-187. (U.S. Department of Commerce: Washington, DC.) Tuljapurkar S. (1990). 'Population dynamics in variable environments.' (SpringerVerlag: New York.) 136 Venables W. N., Ripley B. D. (1997). 'Modern Applied Atatistics with S-plus.' (Springer: New York.) Vignaux M. (1994). Catch per unit effort (CPUE) analysis of west coast South Island Cook Strait spawning hoki fisheries, 1987–93. NZ Fisheries Association Research Document 94/11. Walker T. I. (1998). Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Marine and Freshwater Research 49, 553-572. Walsh W. A., Kleiber P. (2001). Generalized additive model and regression tree analyses of blue shark (Prionace glauca) catch rates by the Hawaii-based commercial longline fishery. Fisheries Research 53, 115-131. Walters C. (2003). Folly and fantasy in the analysis of spatial catch rate data. Canadian Journal of Fisheries and Aquatic Sciences 60, 1433-1436. Ward P., Myers R. A. (2005). Shifts in open-ocean fish communities coinciding with the commencement of commercial fishing. Ecology 86, 835-847. Wise J. P., Le Guen J. C. (1969). The Japanese Atlantic Long - Line Fishery, 19561963. In 'Symposium on the Oceanography and Fisheries Resources of the Tropical Atlantic'. Paris. (UNESCO) Wood C. C., Ketchen K. S., Beamish R. J. (1979). Population dynamics of spiny dogfish (Squalus acanthias) in British Columbia waters. Journal of the Fisheries Research Board of Canada 36, 647-656. Xiao Y. S. (1996). A framework for evaluating experimental designs for estimating rates of fish movement from tag recoveries. Canadian Journal of Fisheries and Aquatic Sciences 53, 1272-1280. 137 APPENDIX A. Supplementary information of Chapter 2. Equations of the indirect methods used to estimate the instantaneous coefficient of natural mortality M (hence natural survivorship, s) for the blue shark, Prionace glauca. 1) Hoenig (1983), ln(M ) 1.46 1.01(ln tmax ) where M is the coefficient of instantaneous natural mortality and tmax is the maximum age. 2) Pauly (1980) log(M ) 0.0066 0.279log( L ) 0.6543log( K ) 0.4634log(T ) where L and K are the asymptotic length and the growth coefficient parameters of the von Bertalanffy growth function, respectively, and T is the mean water temperature (in degrees Celsius). 3) Chen and Watanabe (1989) M (t ) K 1 e K ( t t0 ) , t tm 138 M (t ) K , t tm a0 a1 (t tm ) a2 (t tm ) 2 where, a0 1 e K (tm t0 ) a1 Ke K (tm t0 ) 1 a2 K 2 e K (tm t0 ) 2 and, tm 1 ln(1 e K (t0 ) ) t0 K K and t 0 are parameters of the von Bertalanffy growth function. 4) Peterson and Wroblewski (1984) M w 1.92 w0.25 where w is weight in kilograms. 5) Jensen (1996) – age at maturity method M 1.65 xm where xm is the age at first maturity. 6) Jensen (1996) – growth coefficient method M 1.65( K ) 139 where K is the growth parameter of the von Bertalanffy growth function. Matrix algebra used to determine the net reproductive rate, R0 Net reproductive rate (R0) was determined following the matrix algebra steps presented in Caswell (2001). The parameter represents the mean number of offspring by which a pup will be replaced by the end of its life. In other words, it is the rate by which the population increases from one generation to the next. R0 is the dominant eigenvalue of the R matrix, defined as R = F*N in which, F* is the fertility matrix giving the expected number of offspring produced per time step (denoted with a superscripted asterisk to avoid confusion with F, the coefficient of fishing mortality). It is obtained from decomposition of the Leslie matrix ( M ). It has the same dimensions and the first row elements describing reproduction in M . N is the fundamental matrix giving the expected number of time steps spent in each transient state (age-class), defined as N (I - T)1 140 in which, I is the identity matrix with the same dimensions as M T is the matrix describing the survival transitions resulting from decomposition of the Leslie matrix. It has the same dimensions and survival terms as M 141 APPENDIX B – Supplementary information of Chapter 3 Description of data sources Fishery-dependent (observer) data Data from commercial pelagic longline fisheries operating in the western North Atlantic are available from national fishery observer programs. A chronogram which shows the temporal coverage of U.S. observer programs is shown in Table B.1a. U.S. observers were deployed aboard Japanese tuna longliners fishing in the U.S. Economic Exclusive Zone from 1978 to 1988 (Lopez et al. 1979). Between 1985 and 1990, observers and research scientists were opportunistically deployed on a small number of fishing trips aboard U.S. vessels. After 1990, observers were deployed on permitted U.S. swordfish and tuna vessels randomly selected by the U.S. Pelagic Observer Program of the Southeast Fisheries Science Center of the National Marine Fisheries Service (Lee et al. 1996; Beerkircher et al. 2003). Fishery- independent (survey) data Information was recovered from fishery-independent longline surveys conducted in the western North Atlantic under various research programs (Table B.1b). Between 1957 and 1970, the U.S. Bureau of Commercial Fisheries (BCF) coordinated joint research cruises with the Woods Hole Oceanographic Institute (WHOI) to investigate tuna and swordfish longline fishery potential in the western North Atlantic (Wilson and Barlett 1967). In the late fifties, the swordfish harpoon 142 fishery was dominated by Canadian vessels. The Canadian Department of Fisheries and Oceans (DFO) initiated longline research cruises in 1961, continued them annually through 1972, and occasionally after that until 1990. At the same time (1961), the U.S. Bureau of Sport Fisheries and Wildlife (BSFW), initiated longline cruises out of the Sandy Hook Marine Laboratory in New Jersey to gather life-history information on coastal sharks in response to public concerns about shark attacks on humans (Casey 1985). This survey and related life-history oriented shark research shifted to offshore stations by the mid 1960s, where it complemented the offshore tuna and swordfish surveys (BCF, WHOI and DFO). In 1970, the National Marine Fisheries Service (NMFS) was established under the U.S. Department of Commerce and the shark survey and life-history research continued at the NMFS’s Narragansett Laboratory, Rhode Island, with a primary emphasis on investigation blue and mako sharks migration patterns and biological studies. A fixed station grid survey design utilizing bottom longline gear was implemented in the late 1980’s in response to management concern about the status of large coastal sharks and the expansion of coastal shark targeted fishing effort. Table B.1. Chronogram of the pelagic longline data sources investigated: a) fishery-independent, historical research cruise sources; and, b) fishery-dependent, observer programs on commercial fisheries. Year Data sources 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 00 a) Observer programs NEFSC-PLOP 10 48 ## ## ## ## SEFSC-PLOP 25 80 18 15 23 ## 76 ## ## US-JPN ## ## ## ## ## ## ## ## ## ## ## b) Reseach cruises BCF-WHOI 35 25 27 21 17 26 31 57 24 12 BSFW-SHML CN-DFO 6 10 14 13 21 25 18 12 22 34 36 32 20 20 1 25 49 NARR 38 29 5 9 19 33 64 75 46 49 23 32 24 46 48 NARR-comm 10 NARR-NE 7 7 3 10 26 10 56 8 19 14 2 10 5 16 8 Legend: Data sources Description a) Observer programs SEFSC-PLOP Southeast Fisheries Science Center, Pelagic Longline Observer Program NEFSC-PLOP Northeast Fisheries Science Center, Pelagic Longline Observer Program Dom-opport. Opportunistic deployments aboard commercial vessels US Obs- JPN U.S. observers deployed aboard Japanese vessels b) Research cruise programs BCF-WHOI Cooperative cruises by the Bureau of Commercial Fisheries and the Woods Hole Oceanographic Institution, U.S. BSFW-SHML Bureau of Sport Fisheries and Wildlife, Sandy Hook Marine Laboratory, U.S. NARR National Marine Fisheries Service, Narragansett Laboratory, U.S. NARR-NE Cooperative cruises between NMFS Narragansett Laboratory and other institutions in the northeast U.S. NARR-comm Opportunistic deployments of NMFS Narragansett laboratory scientists aboard volunteer commercial vessels, U.S. CN-DFO Department of Fisheries and Oceans, Canada 14 3 144 Table B.2. Model selection tables. a) binomial error GLM for the proportion of the non-zero sets. Two error assumptions were investigated for the mean catch rate (CPUE) of the positive sets: b) lognormal, c) gamma. d) Negative binomial for the count data. The AIC statistic and the proportion of the total deviance are presented for the models selected for each individual target data set and the reconciled data. a) Proportion of positive sets - binomial Target species Researh cruises TUN SWO PLSHK - all data PLSHK - without Geronimo PLSHK - Geronimo Observer programs TUN (JPN) TUN (US) SWO (US) Reconciled Final model % of dev. year + trimester year + setSzCat1 year + areaNMFS year year + areaNMFS + bottomDepthCat1 7.17 27.57 13.34 14.49 22.12 year + trimester + areaNMFS + rigDepthCat year + trimester + setSzCat1 + lStickCat1 year + trimester + areaNMFS 6.59 11.65 17.75 year + targetID + trimester + areaNMFS + bottomDepthCat1 + setSzCat1 + lStickCat1 + rigDepthCat 9.26 b ) CPUE of positive sets - lognormal Target species Researh cruises TUN SWO PLSHK - all data PLSHK - without Geronimo PLSHK - Geronimo Observer programs TUN (JPN) TUN (US) SWO (US) Reconciled Final model % of dev. year + startSetCat1 + setSzCat1 year + trimester + areaNMFS + bottomDepthCat1 + setSzCat1 year + trimester + areaNMFS + bottomDepthCat1 + setSzCat1 year + areaNMFS + bottomDepthCat1 + startSetCat1 + setSzCat1 year + areaNMFS + bottomDepthCat1 41.10 50.61 54.68 42.3244 37.41 year + trimester + areaNMFS + bottomDepthCat1 + startSetCat1 + setSzCat1 + rigDepthCat year + trimester + areaNMFS + startSetCat1 + setSzCat1 year + trimester + areaNMFS + startSetCat1 + setSzCat1 + lStickCat1 28.79 40.3846 year + targetID + trimester + areaNMFS + bottomDepthCat1 + setSzCat1 + lStickCat1 + rigDepthCat 6.62 57.00 c) CPUE of positive sets - gamma Target species Researh cruises TUN SWO PLSHK - all data PLSHK - without Geronimo PLSHK - Geronimo Observer programs TUN (JPN) TUN (US) SWO (US) Reconciled Final model % of dev. year + startSetCat1 + setSzCat1 year + trimester + areaNMFS + bottomDepthCat1 + setSzCat1 year + trimester + areaNMFS + bottomDepthCat1 + startSetCat1 + setSzCat1 year + trimester + areaNMFS + bottomDepthCat1 + startSetCat1 + setSzCat1 year + trimester + areaNMFS + bottomDepthCat1 42.41 58.62 53.15 46.24 37.91 year + trimester + areaNMFS + setSzCat1 + rigDepthCat year + trimester + areaNMFS + startSetCat1 + setSzCat1 + lStickCat1 year + trimester + areaNMFS + startSetCat1 + setSzCat1 + lStickCat1 9.92 36.86 38.22 year + targetID + trimester + areaNMFS + bottomDepthCat1 + setSzCat1 + lStickCat1 + rigDepthCat 71.66 d) Fish counts - negative binomial Target species Researh cruises TUN SWO PLSHK - all data PLSHK - without Geronimo PLSHK - Geronimo Observer programs TUN (JPN) TUN (US) SWO (US) Reconciled Final model % of dev. year + startSetCat1 + setSzCat1 year + trimester + bottomDepthCat1 + setSzCat1 year + trimester + areaNMFS + bottomDepthCat1 + startSetCat1 + setSzCat1 year + areaNMFS + bottomDepthCat1 + setSzCat1 year + trimester + areaNMFS + bottomDepthCat1 11.98 32.41 29.20 11.30 21.32 year + trimester + bottomDepthCat1 + setSzCat1 + rigDepthCat year + trimester + areaNMFS + bottomDepthCat1 + startSetCat1 year + trimester + areaNMFS + bottomDepthCat1 + setSzCat1 + lStickCat1 4.39 9.34 23.44 year + targetID + trimester + areaNMFS + bottomDepthCat1 + setSzCat1 + lStickCat1 + rigDepthCat 54.72 145 Figure B.1. Diagnostic plots for the lognormal model on the catch rates (CPUE) of the positive sets for each of the target species dataset. Left: qq-plots; Middle: diagnostics on systematic departures from model assumptions; Right: diagnostic on the performance of the variance function. 146 Figure B.2. Diagnostic plots for the gamma model on the catch rates (CPUE) of the positive sets for each of the target species dataset. Left: diagnostics on systematic departures from model assumptions; Right: diagnostic on the performance of the variance function. 147 Figure B.3. Diagnostic tests for the negative binomial model on the blue shark count data for each of the target species dataset. Left: diagnostics on systematic departures from model assumptions; Right: diagnostic on the performance of the variance function. 148 Figure B.4. Delta-lognormal indices for the individual target data sets and reconciled data. The nominal CPUE are also presented for the catch rates of the positive sets. a) Binomial models on the proportion of the noz-zero sets; b) Lognormal models on the CPUE of the noz-zero sets; c) Delta-lognormal indices (product between a and b). 149 Figure B.5. Delta-gamma indices for the individual target data sets and reconciled data. The nominal CPUE are also presented for the catch rates of the positive sets. a) Binomial models on the proportion of the noz-zero sets; b) Gamma models on the CPUE of the noz-zero sets; c) Delta-lognormal indices (product between a and b). 150 Figure B.6. Negative binomial indices based on the count data for each of the individual target data sets and reconciled data. 151 APPENDIX C – Supplementary information of Chapter 4. Table C.1. Time series of annual tag releases and recoveries by the Cooperative Shark Tagging Program (CSTP) in different geographical regions of the North Atlantic Ocean: Northwest (NW), Southwest (SW), Northeast (NE) and the Southeast (SE). Year 1965 1966 1967 1968 1969 1970 1971 1972 1973 1974 1975 1976 1977 1978 1979 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 Totals % Tag releases by geographical region NW SW NE SE 140 0 0 0 273 0 0 0 58 0 0 0 19 0 0 0 6 0 0 0 316 0 0 0 344 0 42 0 742 1 48 0 213 0 75 0 418 0 54 0 567 1 6 0 994 0 27 0 2511 0 14 0 2681 1 8 0 3154 0 11 0 2732 4 7 0 2494 6 42 214 2038 0 1 375 3233 0 2 76 1606 0 8 277 4243 53 2 84 2636 0 109 0 2375 8 62 0 2984 0 16 0 2328 2 3 0 2375 1 17 0 3699 5 70 0 5046 49 69 4 4293 86 96 1 4099 38 107 14 4820 51 91 4 5916 19 92 3 5801 24 38 1 3511 11 23 0 3016 3 25 1 2419 11 91 0 1863 9 208 0 1921 15 110 0 1796 1 496 2 1974 4 114 4 91,654 403 2,184 1,060 0.962 0.004 0.023 0.011 All 140 273 58 19 6 316 386 791 288 472 574 1,021 2,525 2,690 3,165 2,743 2,756 2,414 3,311 1,891 4,382 2,745 2,445 3,000 2,333 2,393 3,774 5,168 4,476 4,258 4,966 6,030 5,864 3,545 3,045 2,521 2,080 2,046 2,295 2,096 95,301 1.000 Tag recoveries by geographical region NW SW NE SE 1 0 0 0 7 0 0 0 4 0 0 0 0 0 0 0 0 1 0 0 3 0 0 0 8 1 0 0 13 0 0 0 17 0 0 0 12 1 0 1 23 1 1 0 41 0 0 0 85 2 0 0 147 6 2 1 102 0 1 0 77 7 0 2 90 4 4 5 45 8 2 7 67 2 1 1 39 4 4 0 140 5 7 2 108 4 4 3 52 5 6 1 109 4 8 0 107 8 4 1 101 9 12 0 152 7 7 1 210 12 9 0 260 14 11 4 269 9 21 5 238 14 16 6 359 8 13 10 417 19 25 17 419 26 23 19 321 18 27 20 338 21 33 13 222 14 34 7 197 13 25 6 168 1 15 5 142 2 41 6 5,110 250 356 143 0.872 0.043 0.061 0.024 All 1 7 4 0 1 3 9 13 17 14 25 41 87 156 103 86 103 62 71 47 154 119 64 121 120 122 167 231 289 304 274 390 478 487 386 405 277 241 189 191 5,859 1.000 Table C.2. Sensitivity analyses of the movement parameters to different scenarios about the tag shedding rate ( ) and the tag reporting rates ( ) of major fisheries. The different model cases investigated are defined in Table 4.4. from to Model case cv6 M7 M1 cv1 M2 cv2 M3 cv3 M4 cv4 M5 cv5 M6 cv7 M8 cv8 M9 cv9 M10 cv10 M11 cv11 M12 cv12 NW SW NE 0.60 0.38 0.02 0.02 0.04 0.15 0.61 0.38 0.02 0.02 0.04 0.15 0.60 0.38 0.02 0.02 0.04 0.15 0.62 0.36 0.02 0.02 0.04 0.15 0.62 0.36 0.02 0.02 0.04 0.15 0.68 0.30 0.02 0.02 0.06 0.16 0.68 0.30 0.02 0.02 0.05 0.16 0.71 0.27 0.02 0.02 0.06 0.16 0.79 0.19 0.02 0.02 0.10 0.16 0.80 0.18 0.02 0.02 0.11 0.16 0.79 0.19 0.02 0.02 0.10 0.16 0.83 0.15 0.03 0.02 0.13 0.16 SW NW SE 0.90 0.03 0.07 0.03 0.25 0.45 0.90 0.04 0.07 0.04 0.25 0.45 0.90 0.03 0.06 0.03 0.25 0.45 0.88 0.04 0.08 0.04 0.25 0.45 0.88 0.04 0.08 0.04 0.25 0.45 0.82 0.07 0.11 0.06 0.27 0.42 0.83 0.06 0.10 0.06 0.26 0.42 0.80 0.08 0.12 0.07 0.27 0.41 0.70 0.11 0.18 0.11 0.33 0.36 0.68 0.12 0.20 0.11 0.33 0.35 0.69 0.12 0.19 0.11 0.32 0.36 0.63 0.13 0.24 0.13 0.36 0.34 NE SE NW 0.68 0.15 0.17 0.07 0.28 0.26 0.69 0.15 0.16 0.07 0.29 0.26 0.69 0.15 0.16 0.07 0.28 0.26 0.69 0.15 0.16 0.07 0.29 0.26 0.69 0.15 0.16 0.07 0.29 0.26 0.71 0.14 0.15 0.07 0.29 0.27 0.71 0.14 0.15 0.07 0.29 0.27 0.71 0.14 0.15 0.07 0.29 0.27 0.72 0.14 0.15 0.07 0.29 0.27 0.72 0.13 0.14 0.07 0.30 0.28 0.72 0.14 0.14 0.07 0.30 0.28 0.73 0.13 0.14 0.07 0.30 0.28 SE SW NE 0.34 0.46 0.20 0.55 0.44 0.55 0.36 0.41 0.23 0.54 0.52 0.55 0.33 0.45 0.22 0.56 0.44 0.55 0.36 0.42 0.22 0.54 0.51 0.56 0.36 0.42 0.22 0.54 0.51 0.56 0.43 0.30 0.27 0.47 0.80 0.51 0.40 0.34 0.26 0.49 0.65 0.51 0.42 0.31 0.27 0.48 0.73 0.51 0.42 0.27 0.30 0.47 0.82 0.46 0.43 0.23 0.34 0.46 1.00 0.44 0.41 0.27 0.32 0.47 0.81 0.44 0.40 0.27 0.33 0.49 0.81 0.44 NW SW NE SE 15 2 Table C.3. Sensitivity analyses of the instantaneous fishing mortality rates (F) to different scenarios about the tag shedding rate ( ) and the tag reporting rates ( ) of major fisheries. The different scenarios investigated are identified in Table 4.4. Model case cv6 M7 cv7 M8 cv8 M9 cv9 M10 cv10 M11 cv11 M12 cv12 0.028 0.003 0.001 0.049 0.018 0.000 0.001 0.001 0.100 0.11 0.29 0.55 0.13 0.25 0.66 1.01 0.08 0.03 0.00 0.00 0.05 0.01 0.00 0.00 0.00 0.09 0.11 0.29 0.55 0.13 0.25 0.66 1.01 0.08 0.05 0.01 0.00 0.05 0.02 0.00 0.00 0.00 0.13 0.11 0.29 0.55 0.13 0.25 0.66 1.01 0.08 0.03 0.00 0.00 0.03 0.02 0.00 0.00 0.00 0.08 0.11 0.29 0.54 0.12 0.25 0.66 1.01 0.09 0.03 0.00 0.00 0.05 0.02 0.00 0.00 0.00 0.10 0.11 0.29 0.55 0.12 0.25 0.66 1.01 0.09 0.03 0.00 0.00 0.05 0.01 0.00 0.00 0.00 0.09 0.11 0.29 0.55 0.12 0.25 0.66 1.01 0.08 0.06 0.01 0.00 0.05 0.02 0.00 0.00 0.00 0.14 0.11 0.29 0.54 0.12 0.25 0.66 1.00 0.08 0.48 0.35 0.47 0.40 1.05 0.57 0.22 0.002 0.004 0.007 0.013 0.002 0.003 0.032 0.48 0.35 0.47 0.40 1.05 0.57 0.22 0.00 0.00 0.00 0.01 0.00 0.00 0.02 0.48 0.35 0.47 0.40 1.04 0.57 0.23 0.00 0.00 0.01 0.02 0.00 0.00 0.04 0.49 0.35 0.47 0.41 1.04 0.57 0.23 0.01 0.00 0.01 0.03 0.00 0.00 0.06 0.50 0.36 0.47 0.42 1.04 0.57 0.26 0.01 0.01 0.01 0.03 0.01 0.01 0.07 0.51 0.36 0.47 0.42 1.04 0.57 0.26 0.01 0.01 0.01 0.01 0.00 0.01 0.04 0.50 0.36 0.48 0.42 1.04 0.57 0.24 0.02 0.01 0.02 0.04 0.01 0.01 0.10 0.52 0.37 0.48 0.43 1.04 0.58 0.27 0.11 0.04 0.02 0.01 0.03 0.19 0.29 0.57 0.65 1.10 0.91 0.25 0.113 0.037 0.016 0.010 0.029 0.204 0.29 0.57 0.65 1.10 0.91 0.25 0.11 0.02 0.02 0.01 0.03 0.19 0.29 0.57 0.65 1.09 0.91 0.25 0.11 0.04 0.02 0.01 0.03 0.20 0.29 0.57 0.65 1.09 0.91 0.25 0.07 0.04 0.02 0.01 0.03 0.16 0.28 0.57 0.64 1.09 0.90 0.27 0.13 0.04 0.02 0.01 0.03 0.22 0.28 0.57 0.65 1.09 0.90 0.25 0.13 0.02 0.02 0.01 0.03 0.21 0.28 0.57 0.65 1.09 0.90 0.25 0.13 0.03 0.02 0.01 0.03 0.22 0.28 0.57 0.65 1.09 0.90 0.25 0.04 0.11 0.00 0.02 0.17 0.53 0.54 0.26 0.71 0.44 0.052 0.111 0.000 0.019 0.182 0.53 0.54 0.26 0.71 0.44 0.05 0.05 0.00 0.02 0.12 0.50 0.54 0.27 0.69 0.40 0.05 0.11 0.00 0.02 0.18 0.49 0.53 0.27 0.69 0.41 0.04 0.12 0.00 0.02 0.18 0.44 0.52 0.28 0.67 0.40 0.08 0.12 0.00 0.03 0.22 0.44 0.52 0.29 0.67 0.37 0.07 0.05 0.00 0.02 0.15 0.44 0.52 0.28 0.67 0.35 0.09 0.12 0.00 0.03 0.23 0.43 0.52 0.30 0.66 0.36 Fishery M1 cv1 M2 cv2 M3 cv3 M4 cv4 M5 cv5 M6 NA_sport_NWNA US_comm_NWNA Canada_comm_NWNA Spain_comm_NWNA Japan_comm_NWNA Portugal_comm_NWNA US_sci - NWNA Pooled_comm_NWNA mean_NWNA 0.03 0.00 0.00 0.02 0.02 0.00 0.00 0.00 0.07 0.11 0.29 0.55 0.13 0.25 0.66 1.01 0.09 0.03 0.00 0.00 0.05 0.02 0.00 0.00 0.00 0.09 0.11 0.29 0.55 0.13 0.25 0.66 1.01 0.09 0.03 0.00 0.00 0.05 0.01 0.00 0.00 0.00 0.08 0.11 0.29 0.55 0.13 0.25 0.66 1.01 0.09 0.05 0.00 0.00 0.05 0.02 0.00 0.00 0.00 0.13 0.11 0.29 0.55 0.13 0.25 0.66 1.01 0.08 0.05 0.00 0.00 0.05 0.02 0.00 0.00 0.00 0.13 0.11 0.29 0.55 0.13 0.25 0.66 1.01 0.08 US_comm_SWNA Spain_comm_SWNA Japan_comm_SWNA Venezuela_comm_SWNA Pooled_sport_SWNA Pooled_comm_SWNA mean_SWNA 0.00 0.00 0.00 0.01 0.00 0.00 0.02 0.47 0.34 0.47 0.40 1.05 0.56 0.24 0.00 0.00 0.00 0.01 0.00 0.00 0.02 0.47 0.35 0.47 0.40 1.04 0.57 0.23 0.00 0.00 0.00 0.00 0.00 0.00 0.01 0.47 0.34 0.47 0.40 1.05 0.57 0.23 0.00 0.00 0.01 0.01 0.00 0.00 0.02 0.48 0.35 0.47 0.40 1.05 0.57 0.22 0.00 0.00 0.01 0.01 0.00 0.00 0.02 Spain_comm_NENA Japan_comm_NENA Portugal_comm_NENA Pooled_sport_NENA Pooled_comm_NENA mean_NENA 0.05 0.04 0.01 0.01 0.03 0.15 0.28 0.57 0.65 1.10 0.91 0.28 0.11 0.04 0.01 0.01 0.03 0.19 0.29 0.57 0.65 1.10 0.91 0.25 0.11 0.02 0.02 0.01 0.03 0.18 0.29 0.57 0.65 1.10 0.91 0.26 0.11 0.04 0.02 0.01 0.03 0.19 0.29 0.57 0.65 1.10 0.91 0.25 Spain_comm_SENA Japan_comm_SENA Venezuela_comm_SENA Pooled_comm_SENA Mean_SENA 0.02 0.11 0.00 0.02 0.15 0.53 0.54 0.27 0.70 0.46 0.04 0.11 0.00 0.02 0.17 0.53 0.54 0.27 0.70 0.44 0.04 0.04 0.00 0.02 0.11 0.53 0.55 0.30 0.70 0.42 0.04 0.11 0.00 0.02 0.17 0.53 0.54 0.26 0.71 0.44 15 3 154 Figure C.1. Inter-regional movements of blue sharks observed by the Cooperative Shark Tagging Project (CSTP) in the North Atlantic Ocean. 155 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.2. Blue shark tag-recoveries by the U.S.-sport fishery, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (19652004). 156 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.3. Blue shark tag-recoveries by the U.S.-commercial fishery, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (19652004). 157 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.4. Blue shark tag-recoveries by the Canada-commercial fishery, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (19652004). 158 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.5. Blue shark tag-recoveries by the Japan-commercial fishery, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (19652004). 159 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.6. Blue shark tag-recoveries by the Spain-commercial fishery, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (19652004). 160 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.7. Blue shark tag-recoveries by the Venezuela-commercial fishery, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (1965-2004). 161 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.8. Blue shark tag-recoveries by the Portugal-commercial fishery, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (1965-2004). 162 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.9. Blue shark tag-recoveries by U.S.-scientific cruises, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (19652004). 163 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.10. Blue shark tag-recoveries by the others -commercial fishery, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (19652004). 164 100° 90° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 60° 50° 50° 40° 40° 30° 30° 20° 20° 10° 0° Ü 90° 10° 80° 70° 60° 50° 40° 30° 20° 10° 0° 10° 20° Figure C.11. Blue shark tag-recoveries by the others-sport fishery, archived by the Cooperative Shark Tagging Program (CSTP) in the North Atlantic Ocean (19652004) 4 4 Sport-N. America (1) 2 NW 0 4 0 4 Relative fishing effort 2 0 0 4 Com. - US (3) Com. - Portugal (15) 2 2 2 0 0 0 0 4 4 Com. - Japan (11) 4 Com. - Venez. (17) 4 Sport - others (20) Comm. - others (24) 4 2 2 2 2 2 0 0 0 0 0 0 8 4 4 4 4 Com. - Japan (12) Com. - Portugal (16) Sport - others (21) Com. - others (25) 4 2 2 2 2 0 0 0 0 0 40 8 4 4 Com. - Spain (8) SE 4 Com. - Japan (10) 2 Com. - Spain (6) Com. - Spain (7) NE 4 Com. - Spain (5) Com. - others (23) 2 8 4 Com. - Canada (4) 2 Survey - US (19) SW 4 Com. - US (2) 20 4 0 1960 Com. - Japan (13) 2 0 1980 2000 1960 Com. - Venez. (18) 2 0 1980 2000 1960 Com. - others (26) 0 1980 2000 1960 1980 2000 Years Figure C.12. Relative fishing effort for each fishery. 16 5 166 VITA Alexandre Aires-da-Silva was born in Maputo, Mozambique. He grew up in the city of Lisbon, Portugal, his country of citizenship. He earned a Licenciatura (Bachelor) degree in Marine Biology of Fisheries at Universidade do Algarve, Faro, Portugal (1996). After that, he worked as a fishery scientist at Departamento de Oceanografia e Pescas, Univerisdade dos Açores, in the Azores, Portugal (1996-2000). In 2000, Alexandre enrolled as a Fulbright graduate student at the School of Aquatic and Fishery Sciences, University of Washington, Seattle, Washington, U.S. He now takes a Senior Scientist position with the Inter-American Tropical Tuna Commission, in La Jolla, California, U.S. In 2008, Alex graduated from the University of Washington with a Ph.D. from the School of Aquatic and Fishery Sciences.