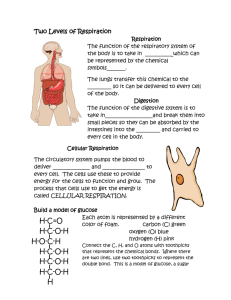
Aim: To investigate the anaerobic respiration rate of yeast in different
advertisement

Aim: To investigate the anaerobic respiration rate of yeast in different concentration of glucose solution Biological Principle: 1.In the absence of oxygen, yeast will undergo anaerobic respiration .,Yeast converts glucose into ethanol and carbondioxide. So, the carbondioxide given out reflect the rate of anaerobic respiration. 2.Glucose was fermented because this sugar can pass rapidly into the cell and enter directly into metabolic pathways Apparatus: Yeast Glucose powder Ice Conical flask Bunsen Burner Co2 Sensor Paraffin oil Beaker Procedure: 1. 4 grams of yeast was measured by the electronic balance. 2. Glucose solution was prepared in a beaker 3. Different concentration of glucose solution was prepared as follows: Percentage of glucose solution 3% 6% 9% 0% (control set-up) Volume of water added (cm3) 145.5 141 136.5 150 Mass of glucose added (g) 4.5 9 13.5 0 4. The glucose solution of 3% was boiled under the Bunsen flame to evaporate any dissolve oxygen in the solution. 5. This glucose solution was cooled down by the Ice to prevent the heat produced killed the yeast during the fermentation. 6. The carbondioxide sensor was connected with the Data Logger. 7. Glucose solution was then mixed with the yeast in the reagent bottle under the water bath with temperature 30 oC. Water bath was prepared to maintain the temperature as heat produced will affect the reaction during anaerobic respiration. 8. A layer of oil was added on the top of the solution to prevent gas trapped in the solution. 9. The Cardon dioxide Sensor sealed the reagent bottle of the mixed solution 10. The reagent bottle was put under the water bath for 10 minutes. 11. The amount of carbondioxide was recorded at each 5 seconds interval. 12. Steps 4 to 11 are repeated but the concentration of glucose solution is changed to 6%, 9% and 0% respectively. The control set up(0%) is to correct any experimental error arised from any endogenous substrate(glucose) already present in yeast.