Activity 4.2.4 Just Passing Through
advertisement

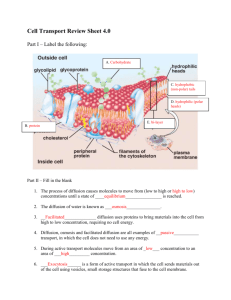
Name _________________________________________________ Date____________ Activity 4.2.4 – Just Passing Through Purpose Plant uptake of water is sometimes referred to as “absorption” of water. Some people assume that roots are sponges that soak up water and the various dissolved nutrients. This is a misconception. It is important for you to understand the scientific explanation for how this process really happens in plants. The uptake of water and dissolved nutrients are cellular processes called diffusion and osmosis. Molecules are selectively allowed to pass through membranes. For molecules to pass through the membrane, they must be small and of differing concentrations. The result is diffusion of substances between the solutions on each side of the membrane until the concentration of substances is equal. When the concentration of water on either side of the cell membrane is unequal, osmosis or the passing of water through the membrane occurs. You will simulate the processes of diffusion and osmosis, which cells depend on to take in essential nutrients. Materials Day 1: Per pair of students: 4 jars with lids Potato Permanent marker Distilled water 3 % salt solution 5 % salt solution 10% salt solution Knife Per class: Electronic balance Procedure You and your partner will monitor the diffusion and osmosis of substances by weighing a potato left in different salt solutions. This is similar to the process that cells use to transfer material through a cell membrane. Follow the procedures below and record necessary evidence of diffusion and osmosis. The National Council for Agricultural Education – CASE – Copyright 2010 Plant – Unit 4 – Lesson 4.2 – Activity 4.2.4 – Just Passing Through – Page 1 Day 1 1. Write your group initials on each beaker. 2. Label your three beakers 0%, 3%, 5%, and 10% respectively. 3. Carefully using your knife, cut four 1 cm x 1 cm x 5 cm strips of potato. 4. Place one strip in each beaker. 5. Find the mass of each strip using an electronic balance. Make sure to zero the balance before you weigh. Return the strip to its beaker after you weight it. 6. Record the masses in Table 1. 7. Pour the correct solution into each jar until the potato piece is completely covered. Cover with a lid. 8. Place your jars on the lab cart and clean up your lab station. 9. Discuss the question below as a group and write an answer in the box. Hypothesis: What do you think will happen to each potato piece? 0% salt solution (distilled water): 3% salt solution: 5% salt solution: 10% salt solution: Day 2 10. Obtain your jars from yesterday. 11. Remove the potato from the 0% jar and blot dry with a paper towel. 12. Quickly weigh the piece using the electronic balance. Make sure to zero the balance before you weigh. 13. Record the mass in Table 1. 14. Determine the change in mass in Table 1. 15. Repeat steps 11-14 for the remaining jars. 16. Make a line graph of your data by graphing the weight difference for each salt solution. 17. Clean up your lab station and answer the question below. Was your hypothesis from yesterday correct? Why or why not? Explain. The National Council for Agricultural Education – CASE – Copyright 2010 Plant – Unit 4 – Lesson 4.2 – Activity 4.2.4 – Just Passing Through – Page 2 Table 1 Solution 0% Mass (g) Day 2 Difference Day 1 Observations Color, Texture, Flexibility 3% 5% 10% Graph 1 (+) Change in mass (g) 0 (-) Salt solution 0 1 2 3 4 5 6 7 8 9 10 The National Council for Agricultural Education – CASE – Copyright 2010 Plant – Unit 4 – Lesson 4.2 – Activity 4.2.4 – Just Passing Through – Page 3 Conclusion 1. Which solution caused the potato to gain the most weight? 2. In this solution, was water moving into or out of the potato cells? How do you know? 3. Which solution caused the potato to lose the most weight? 4. In this solution, was water moving into or out of the potato cells? How do you know 5. Are the salt solutions hypertonic or hypotonic compared to the potato cells? How do you know? List at least two reasons. 6. Look at your line graph. Find the point where the line crosses 0. This is the point where the potato does not gain or lose any more mass. Follow the line down to the bottom axis to see the salt concentration at this point. What is it? 7. Why would it be important to know the optimal salt concentration for potatoes? List two reasons. 8. If you place an animal blood cell in water, the cell will take in water until it bursts. What prevents this from happening in plant cells? 9. In a grocery store, fresh vegetables are often sprayed with fresh water. Explain this using the term “osmosis”. The National Council for Agricultural Education – CASE – Copyright 2010 Plant – Unit 4 – Lesson 4.2 – Activity 4.2.4 – Just Passing Through – Page 4