Diffusion_and_Osmosis.doc
advertisement

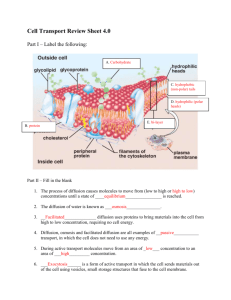
Diffusion and Osmosis Kelli Mulholland September 11, 2010 Abstract: All biological systems are made of cells, which are composed of and are surrounded by a solution. In order for the cells to interact with their environment; a process must happen; either osmosis of diffusion. This movement over the cell membrane occurs to maintain an isotonic or equal mixture of solution inside and outside of the cell. Introduction: Solutions are equally distributed throughout the cell and the cell’s environment. This is a continual movement of molecules through the cell and the cell membrane; achieved by osmosis and diffusion. Osmosis is the movement of molecules down a concentration gradient, with the ability to cross the cellular membrane with the aid of a protein channel imbedded in the membrane. Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration. Due to these passive molecular motions, molecules move randomly until the environment is isotonic. The concentration of the solution is the determinant of the movement of the molecules traveling in or out of the cell. For example, when the environment outside a cell has a lower concentration of dissolved molecules than inside the cell, the solution is hypotonic. When hypo tonicity occurs, water will move from the solution into the cell. Inversely, if the surrounding solution has a higher concentration of dissolved molecules than the cell, the solution is hypertonic. Water will then move from the cell; outward into the surrounding solution, thereby creating an isotonic solution. 1 In this experiment we will observe osmosis or the diffusion of water in a potato placed in a salt water solution. If osmosis occurs, then the potato will have an increase in weight. If diffusion occurs, after an unknown time and concentration, the solution and the potato will reach a level of isotonicity and no more weight gain will occur. In addition, if osmosis does occur, then our control as stated, is the potato in no water or salt solution, then the initial weight of the potato will remain a constant. Materials and Methods: Three sets of four paper cups were obtained. One cup of each set was labeled ‘water’, another set was labeled 1 ml of NaCL, another labeled 5 ml of NaCl and the last set was labeled 10 ml of NaCl. An addition cup was designated as the control and nothing was placed in the cup other than a weighed piece of potato. To the three sets salt and cups, 50 ml of tepid water was added and stirred with a spatula to aid on the dissolving of the salt in the water. In regards to the potato, it was first peeled and the cut to fairly uniform chunks and weighed. After the chunked potatoes were weighed, a single piece was placed in the varied solutions of salt water. After 20 minutes had lapsed, the potatoes were removed from the solution and weighed again, including the potato that was designated at the control. 2 Figure: 1 Table of results. Discussion: After the potato was in the H20, the weight of the potato increased, suggesting that osmosis was carried out. The potato is trying to create and equilibrium in the cup and potato. In turn, after the potato was in the salt solution the potato demonstrated a decrease in weight, thereby allowing the water to flood from the potato into the solution; again trying to obtain equilibrium. After the 20 minute soak time, the weight of the potato in both the water and the salt solution demonstrated a change in weight. The potato designated as the control had no change in weight, suggesting that there were no factors, such as the change in environment that would influence any exchange across the membrane. The overall results are a bit skewed. These errors could have many contributing factors: including poor averaged potato size, percent salt water solution; as the group used a graduated cylinder to measure the salt. Granted, the same 3 amount of salt was used for each trial set, but I am sure that type of measurement isn’t the best tool for the dry materials. Additionally, the third trial set does not demonstrate the same results as the first two; in that all the pieces of potato lost weight, whereas the first two trial sets gained weight. If I were to perform this experiment again, I would use to correct measurement tools, cut the potato more uniformly, making sure the weights were closer to each other and even larger pieces as well. Also, I believe that checking the potatoes at different time intervals would have given us better data for the process of osmosis and equilibrium, observing diffusion. All in all, the theory of osmosis and diffusion were demonstrated in this example, but still had too many factors that possibly didn’t demonstrate the best data results. References: Bio 111 Lab #3 Lab instruction sheet; RMC September 2010. 4