Honey I Shrunk the Carrot Lab
advertisement

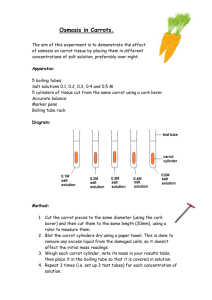
Name: __________________________ Date: _________________ Honey, I Shrunk the Carrots! Observing Osmosis Background Information: Molecules are in constant motion, and tend to move from areas of higher concentrations to areas of lesser concentrations. Diffusion is defined as the movement of molecules from an area of high concentration to an area of low concentration. This is known as a concentration gradient because the molecules are not evenly distributed. The diffusion of water molecules through a selectively permeable membrane is known as osmosis. Selectively permeable means that some molecules can move through the membrane while others cannot. Movement through membranes is called transport. Diffusion and osmosis are passive forms of transport; this means that do not need energy to move areas of high concentration to areas of low concentration. Active transport requires energy to transport molecules from low concentration to high concentration. Think of riding your bike down a hill as passive transport and riding your bike up a hill as active transport. Osmosis is the movement (transport) of water (like the small dots below) through a selectively permeable membrane from an area of high concentration to an area of low concentration. In the diagram below, active transport would be needed to transport the large dots through the membrane from the right side to the left. Review Questions: 1. What is diffusion? ________________________________________________________________________ ________________________________________________________________________ 2. What is osmosis? ________________________________________________________________________ ________________________________________________________________________ 3. What is a concentration gradient? ________________________________________________________________________ ________________________________________________________________________ 4. How are diffusion and osmosis alike? ________________________________________________________________________ ________________________________________________________________________ 5. How are diffusion and osmosis different? ________________________________________________________________________ ________________________________________________________________________ Objective: Students will use carrots as cell models and demonstrate osmosis. Students will measure and observe the changes in the mass and size of the carrot. Materials: Two 250 mL beakers string salt distilled water digital scale 2 baby carrots masking tape pen spoon Carrot Experiment Procedures (Check off each step as you do them): DAY 1: 1. ____ Label the beakers with your names and label one SALT WATER, the other FRESH WATER using masking tape. 2. ____ Fill each beakers with 200mL. 3. ____ Place the filter paper on the scale and “ZERO” it. 4. _____ Measure 15 grams of salt on the scale 5. _____ Add 15 g salt to one beaker and label it "Salt Water". Use the spoon to dissolve the salt. 6. _____Place 1 carrot on the scale and record the starting mass in the table below. 7. _____Tightly tie a piece of string around the carrot in the middle. 8. _____Place this carrot in the "Salt Water" beaker. 9. _____Place the other carrot on the scale and record the starting mass in the table below. 10. _____Tightly tie a piece of string around the carrot in the middle. 11. _____Place this carrot in the "Fresh Water" beaker. 12. ____ Move your beakers to the tray up front. Allow carrots to remain undisturbed for 24 hours. QUESTION: What is the independent variable? ____________________________________________________________________ QUESTION: What is the dependent variable? ____________________________________________________________________ 13. Talk to your group to form a hypothesis for the outcome of this experiment and record it below: RESPOND: If the two carrots are allowed to sit undisturbed over __________________________ (independent variable), then the carrot in the _____________________ beaker will become __________________________ (dependent variable). Results Of Soaking a Carrot in Salt Water or Fresh Water Condition Fresh Water Carrot Salt Water Carrot Starting Mass (grams) Ending Mass (grams) Texture of carrot (firm or soft) Fit of thread (loose or tight) DAY 2: 14. _____ Remove the carrot from the salt water. Observe it and the tightness of the string and record it in the chart above. 15. _____ Remove the string from the carrot and gently dry it. 16. _____ Place the carrot on the scale and record the final mass of the carrot in the chart above as the ending mass. 17. _____ Remove the carrot from the fresh water. Observe it and the tightness of the string and record it in the chart above. 18. _____ Remove the string from the carrot and gently dry it. 19. _____ Place the carrot on the scale and record the final mass of the carrot in the chart above as the ending mass. Conclusion Questions 1. What was the purpose of tying thread on each carrot? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 2. What was the difference in mass of the carrot in Fresh water after the experiment? _____________________________ Did it increase or decrease? ___________________ 3. What was the difference in mass of the carrot in Salt water after the experiment? _____________________________ Did it increase or decrease? ___________________ 4. In which kind of water did the carrot cells lose water? ______________________ 5. How can you tell (explain your evidence)? ________________________________________________________________________ ________________________________________________________________________ 6. In which kind of water did the carrots gain water? _________________________ 7. How can you tell (explain your evidence)? ________________________________________________________________________ ________________________________________________________________________ 8. What do you think would happen to human blood cells if they were placed in a beaker of salt water? Why? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 9. Draw a diagram of each of your carrots in their beakers. Title each diagram label if the carrot is in plain or salt water Draw arrows showing the water moving in or out of the cells of the carrot Application Questions: 1. Your favorite plant has begun to wilt. You feel the soil in the pot and find that it is very dry. You water your plant and about 20 minutes later you discover the plant is standing up straight again. Is this an example of osmosis or diffusion? Explain. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 2. Why do grocery stores spray their fresh produce with water? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 3. If a shipwrecked crew drank salt water, they could die. Explain why. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 4. If a bowl of fresh strawberries is sprinkled with sugar, a few minutes later they will be covered with juice. Explain why this happens. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________