Electrolytes play a vital role in maintaining homeostasis within the

Electrolytes play a vital role in maintaining homeostasis within the body. They help to regulate myocardial and neurological function, fluid balance, oxygen delivery, acid-base balance and much more
. Electrolyte imbalances can develop by the following mechanisms: excessive ingestion; diminished elimination of an electrolyte; diminished ingestion or excessive elimination of an electrolyte. The most common cause of electrolyte disturbances is renal failure .
The most serious electrolyte disturbances involve abnormalities in the levels of sodium , potassium , and/or calcium .
Table of common electrolyte disturbances
Electrolyte Ionic formula Elevation disorder Depletion disorder
Sodium Na
+ hypernatremia hyponatremia
Potassium K + hyperkalemia hypokalemia
Calcium Ca
2+ hypercalcemia hypocalcemia
Magnesium Mg
2+ hypermagnesemia hypomagnesemia
Chloride
Phosphate
Cl
hyperchloremia hypochloremia
PO
4
3hyperphosphatemia hypophosphatemia
Bicarbonate HCO
3
hyperbicarbonatemia hypobicarbonatemia
General Function
Electrolytes are important because they are what cells (especially nerve, heart, muscle) use to maintain voltages across their cell membranes and to carry electrical impulses
(nerve impulses, muscle contractions) across themselves and to other cells.
Kidneys work to keep the electrolyte concentrations in blood constant despite changes in your body. For example, during heavy exercise, electrolytes are lost in sweat, particularly sodium and potassium. These electrolytes must be replaced to keep the electrolyte concentrations of the body fluids constant.
Electrolyte abnormalities and
ECG
changes
Potassium o The most notable feature of hyperkalemia is the "tent shaped" or "peaked"
T wave. Delayed ventricular depolarization leads to a widened QRS o complex and the P wave becomes wider and flatter.
When hyperkalemia becomes severe, the ECG resembles a sine wave as the P wave disappears from view.
1
o In contrast, hypokalemia is associated with flattening of the T wave and the appearance of a U wave. When untreated, hypokalemia may lead to severe arrhythmias.
Calcium o The fast ventricular depolarization and repolarization associated with hypercalcemia lead to a characteristic shortening of the QT interval. o Hypocalcemia has the opposite effect, lengthening the QT interval.
For acidosis referring to acidity of the urine, see renal tubular acidosis .
Acidosis is an increased acidity (i.e. an increased hydrogen ion concentration ). If not further qualified, it usually refers to acidity of the blood plasma .
Acidosis is said to occur when arterial pH falls below 7.35, while its counterpart
( alkalosis ) occurs at a pH over 7.45.
Arterial blood gas analysis and other tests are required to separate the main causes.
The term acidemia describes the state of low blood pH, while acidosis is used to describe the processes leading to these states. Nevertheless, physicians sometimes use the terms interchangeably. The distinction may be relevant where a patient has factors causing both acidosis and alkalosis, where the relative severity of both determines whether the result is a high or a low pH.
The rate of cellular metabolic activity affects and, at the same time, is affected by the pH of the body fluids
General symptoms of acidosis. These usually accompany symptoms of another primary defect (respiratory or metabolic).
2
Metabolic acidosis
Metabolic acidosis is an increased production of metabolic acids, usually resulting from disturbances in the ability to excrete acid via the kidneys . Renal acidosis is associated with an accumulation of urea and creatinine as well as metabolic acid residues of protein catabolism.
An increase in the production of other acids may also produce metabolic acidosis. For example, lactic acidosis may occur from 1) severe (PaO
2
<36mm Hg) hypoxemia causing a fall in the rate of oxygen diffusion from arterial blood to tissues, or 2) hypoperfusion
(e.g. hypovolemic shock) causing an inadequate blood delivery of oxygen to tissues. A rise in lactate out of proportion to the level of pyruvate, e.g. in mixed venous blood, is termed "excess lactate", and may also be an indicator of fermention due to anaerobic metabolism occurring in muscle cells, as seen during strenuous exercise. Once oxygenation is restored, the acidosis clears quickly. Another example of increased production of acids occurs in starvation and diabetic acidosis. It is due to the accumulation of ketoacids ( ketosis ) and reflects a severe shift from glycolysis to lipolysis for energy needs.
Acid consumption from poisoning , elevated levels of iron in the blood, and chronically decreased production of bicarbonate may also produce metabolic acidosis.
Metabolic acidosis is compensated for in the lungs, as increased exhalation of carbon dioxide promptly shifts the buffering equation to reduce metabolic acid. This is a result of stimulation to chemoreceptors which increases alveolar ventilation , leading to respiratory compensation, otherwise known as Kussmaul breathing (a specific type of hyperventilation ). Should this situation persist the patient is at risk for exhaustion leading to respiratory failure .
Mutations to the V-ATPase 'a4' or 'B1' isoforms result in distal renal tubular acidosis, a condition that leads to metabolic acidosis, in some cases with sensorineural deafness.
Arterial blood gases will indicate low pH , low blood HCO
3
, and normal or low PaCO
2
. In addition to arterial blood gas, an anion gap can also differentiate between possible causes.
The Henderson-Hasselbalch equation is useful for calculating blood pH, because blood is a buffer solution . The amount of metabolic acid accumulating can also be quantitated by using buffer base deviation, a derivative estimate of the metabolic as opposed to the respiratory component. In hypovolemic shock for example, approximately 50% of the metabolic acid accumulation is lactic acid, which disappears as blood flow and oxygen debt are corrected.
3
Treatment of uncompensated metabolic acidosis is focused upon correcting the underlying problem. When metabolic acidosis is severe and can no longer be compensated for adequately by the lungs, neutralizing the acidosis with infusions of bicarbonate may be required.
Respiratory acidosis
Respiratory acidosis results from a build-up of carbon dioxide in the blood (hypercapnia) due to hypoventilation . It is most often caused by pulmonary problems, although head injuries , drugs (especially anaesthetics and sedatives ), and brain tumors can cause this acidemia. Pneumothorax , emphysema , chronic bronchitis , asthma , severe pneumonia , and aspiration are among the most frequent causes. It can also occur as a compensatory response to chronic metabolic alkalosis .
One key to distinguish between respiratory and metabolic acidosis is that in respiratory acidosis, the CO
2
is increased while the bicarbonate is either normal (uncompensated) or increased (compensated). Compensation occurs if respiratory acidosis is present, and a chronic phase is entered with partial buffering of the acidosis through renal bicarbonate retention.
However, in cases where chronic illnesses which compromise pulmonary function persist, such as late-stage emphysema and certain types of muscular dystrophy , compensatory mechanisms will be unable to reverse this acidotic condition. As metabolic bicarbonate production becomes exhausted, and extraeneous bicarbonate infusion can no longer reverse the extreme buildup of carbon dioxide associated with uncompensated respiratory acidosis, mechanical ventilation will usually be applied.
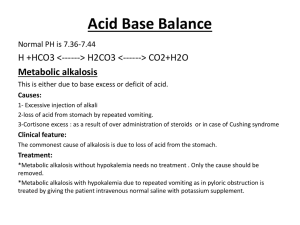
Alkalosis
Alkalosis refers to a condition reducing hydrogen ion concentration of arterial blood plasma ( alkalemia ). Generally alkalosis is said to occur when pH of the blood exceeds
7.45
. The opposite condition is acidosis .
Types
More specifically, alkalosis can refer to:
Respiratory alkalosis
Metabolic alkalosis
Causes
The main cause of respiratory alkalosis is hyperventilation , resulting in a loss of carbon dioxide . Compensatory mechanisms for this would include increased dissociation of the
4
carbonic acid buffering intermediate into hydrogen ions , and the related excretion of bicarbonate ,
[ citation needed ]
both of which would lower blood pH .
Metabolic alkalosis can be caused by prolonged vomiting, resulting in a loss of hydrochloric acid with the stomach content. Severe dehydration , and the consumption of alkali are other causes. It can also be caused by administration of diuretics and endocrine disorders such as Cushing's syndrome . Compensatory mechanism for metabolic alkalosis involve slowed breathing by the lungs to increase serum carbon dioxide, a condition leaning toward respiratory acidosis . As respiratory acidosis often accompanies the compensation for metabolic alkalosis, and vice versa, a delicate balance is created between these two conditions.
Complications
Metabolic alkalosis is usually accompanied with hypokalemia , causing e.g. muscular weakness, myalgia , and muscle cramps (owing to disturbed function of the skeletal muscles), and constipation (from disturbed function of smooth muscles).
It may also cause hypocalcemia . As the pH of blood increases, the protein in the blood becomes more ionised into anions. This causes the free calcium present in blood to bind strongly with protein. If severe, it may cause tetany ( alkalotic tetany ).
Respiratory alkalosis
Respiratory alkalosis is a medical condition in which increased respiration
( hyperventilation ) elevates the blood pH (a condition generally called alkalosis ). It is one of four basic categories of disruption of acid-base homeostasis .
Types
There are two types of respiratory alkalosis: chronic and acute .
Acute respiratory alkalosis occurs rapidly. During acute respiratory alkalosis, the person may lose consciousness where the rate of ventilation will resume to normal.
Chronic respiratory alkalosis is a more long-standing condition. For every 10 mM drop in pCO
2
in blood, there is a corresponding 5 mM of bicarbonate ion drop. The drop of 5 mM of bicarbonate ion is a compensation effect which
5
reduces the alkalosis effect of the drop in pCO
2
in blood. This is termed metabolic compensation.
Mechanism
Respiratory alkalosis generally occurs when some stimulus (see "Causes" below) makes a person hyperventilate. The increased breathing produces increased alveolar respiration, expelling CO
2
from the circulation. This alters the dynamic chemical equilibrium of carbon dioxide in the circulatory system, and the system reacts according to Le
Chatelier's principle . Circulating hydrogen ions and bicarbonate are shifted through the carbonic acid (H
2
CO
3
) intermediate to make more CO
2
via the enzyme carbonic anhydrase according to the following reaction:
The net result of this is decreased circulating hydrogen ion concentration, and thus increased pH (alkalosis). There is also a decrease in ionized blood calcium concentration.
Causes
Respiratory alkalosis may be produced accidentally by doctors ( iatrogenically ) during excessive mechanical ventilation . Other causes include:
psychiatric causes: anxiety , hysteria and stress
CNS causes: stroke , subarachnoid haemorrhage , meningitis drug use: doxapram , aspirin , caffeine and coffee abuse
moving into high altitude areas, where the low atmospheric pressure of oxygen
stimulates increased ventilation lung disease such as pneumonia , where a hypoxic drive governs breathing more than CO
2
levels (the normal determinant)
fever , which stimulates the respiratory centre in the brainstem
pregnancy sexual activity, which may induce excessive breathing due to excitation
Symptoms
Symptoms of respiratory alkalosis are related to the decreased blood carbon dioxide levels, and include peripheral paraesthesiae . In addition, the alkalosis may disrupt calcium ion balance, and cause the symptoms of hypocalcaemia (such as tetany and fainting ) with no fall in total serum calcium levels.
6
Metabolic alkalosis
Metabolic alkalosis is a metabolic condition in which the pH of the blood is elevated beyond the normal range ( 7.35-7.45 ). This is usually the result of decreased hydrogen ion concentration, leading to increased bicarbonate , or alternatively a direct result of increased bicarbonate concentrations.
Causes
There are five main causes of metabolic alkalosis
These can be divided into two categories, depending upon urine chloride levels.
Chloride-responsive (<10 mEq/L)
Loss of hydrogen ions - Most often occurs via two mechanisms, either vomiting or via the kidney. o Vomiting results in the loss of hydrochloric acid with the stomach content.
To make stomach acid, body moves hydrogen ions from blood to the o stomach, causing low hydrogen ions in blood, raising pH of the blood.
Renal losses of hydrogen ions occurs when excess aldosterone induces the retention of sodium and hence the excretion of hydrogen from blood to urine, causing low hydrogen ions in blood, raising pH of the blood.
Contraction alkalosis - This results from a loss of water in the extracellular space which is poor in bicarbonate, typically from diuretic use. Since water is lost while bicarbonate is retained, the concentration of bicarbonate increases blood pH.
Chloride-resistant (>20 mEq/L)
Retention of bicarbonate
Shift of hydrogen ions into intracellular space - Seen in hypokalemia . Due to a low extracellular potassium concentration, potassium shifts out of the cells. In order to maintain electrical neutrality, hydrogen shifts into the cells, raising blood pH.
Alkalotic agents - Alkalotic agents, such as bicarbonate (administrated in cases of peptic ulcer or hyperacidity ) or antacids, administered in excess can lead to an alkalosis.
7
Compensation
Compensation for metabolic alkalosis occurs mainly in the lungs, which retain carbon dioxide (CO
2
) through slower breathing, or hypoventilation ( respiratory compensation ).
CO
2
is then consumed toward the formation of the carbonic acid intermediate, thus decreasing pH. Respiratory compensation, though, is incomplete. The decrease in [H+] suppresses the peripheral chemoreceptors, which are sensitive to pH. But, because respiration slows, there's an increase in Pco2 which would cause an offset of the depression because of the action of the central chemoreceptors which are sensitive to the partial pressure of CO2 in the blood. So, because of the central chemoreceptors, respiration rate would be increased.
Renal compensation for metabolic alkalosis, less effective than respiratory compensation, consists of increased excretion of HCO
3
-
(bicarbonate), as the filtered load of HCO
3
exceeds the ability of the renal tubule to reabsorb it.
Hypokalemia
Hypokalemia (refers to the condition in which the concentration of potassium (K
+
) in the blood is low. Normal serum potassium levels are between 3.5 to 5.0 mEq/L , at least 95% of the body's potassium is found inside cells , with the remainder in the blood. This concentration gradient is maintained principally by the Na+/K+ pump .
Signs and symptoms
Mild hypokalemia is often without symptoms, although it may cause a small elevation of blood pressure , and can occasionally provoke cardiac arrhythmias.
Moderate hypokalemia, with serum potassium concentrations of 2.5-3 mEq/L, may cause muscular weakness, myalgia , and muscle cramps (owing to disturbed function of the skeletal muscles), and constipation (from disturbed function of smooth muscles). With more severe hypokalemia, flaccid paralysis , hyporeflexia , and tetany may result. There are reports of rhabdomyolysis occurring with profound hypokalemia with serum potassium levels less than 2 mEq/L. Respiratory depression from severe impairment of skeletal muscle function is found in many patients.
Some electrocardiographic ( ECG ) findings associated with hypokalemia are flattened T waves and prolongation of the QT interval . The prolonged QT interval may lead to arrhythmias.
8
Hyperkalemia
Hyperkalemia ( hyper high; kalium , potassium; -emia , "in the blood") is an elevated blood level of the electrolyte potassium . Extreme hyperkalemia is a medical emergency due to the risk of potentially fatal abnormal heart rhythms ( arrhythmia ).
Signs and symptoms
Symptoms are fairly nonspecific and generally include malaise , palpitations and muscle weakness ; mild hyperventilation may indicate a compensatory response to metabolic acidosis , which is one of the possible causes of hyperkalemia. Often, however, the problem is detected during screening blood tests for a medical disorder, or it only comes to medical attention after complications have developed, such as cardiac arrhythmia or sudden death .
During the medical history taking, a physician will dwell on kidney disease and medication use (see below), as these are the main causes. The combination of abdominal pain , hypoglycemia and hyperpigmentation , often in the context of a history of other autoimmune disorders , may be signs of Addison's disease , itself a medical emergency.
Diagnosis
In order to gather enough information for diagnosis, the measurement of potassium needs to be repeated, as the elevation can be due to hemolysis in the first sample. The normal serum level of potassium is 3.5 to 5 mEq/L. Generally, blood tests for renal function
( creatinine , blood urea nitrogen ), glucose and occasionally creatine kinase and cortisol will be performed. Calculating the trans-tubular potassium gradient can sometimes help in distinguishing the cause of the hyperkalemia.
In many cases, renal ultrasound will be performed, since hyperkalemia is highly suggestive of renal failure.
Also, electrocardiography (EKG/ECG) may be performed to determine if there is a significant risk of cardiac arrhythmias (see ECG/EKG Findings , below).
Differential diagnosis
Causes include:
Ineffective elimination from the body
Renal insufficiency
Medication that interferes with urinary excretion: o ACE inhibitors and angiotensin receptor blockers
9
o o o o
Potassium-sparing diuretics (e.g. amiloride and spironolactone )
NSAIDs such as ibuprofen , naproxen , or celecoxib
The calcineurin inhibitor immunosuppressants ciclosporin and tacrolimus
The antibiotic trimethoprim o The antiparasitic drug pentamidine
Mineralocorticoid deficiency or resistance, such as: o o
Addison's disease
Aldosterone deficiency , including reduced levels due to the blood thinner, o o heparin
Some forms of congenital adrenal hyperplasia
Type IV renal tubular acidosis (resistance of renal tubules to aldosterone)
Gordon's syndrome (“familial hypertension with hyperkalemia”), a rare genetic disorder caused by defective modulators of salt transporters, including the thiazide-sensitive Na-Cl cotransporter .
Excessive release from cells
Rhabdomyolysis , burns or any cause of rapid tissue necrosis , including tumor lysis syndrome
Massive blood transfusion or massive hemolysis
Shifts/transport out of cells caused by acidosis , low insulin levels, beta-blocker therapy, digoxin overdose, or the paralyzing agent succinylcholine
Excessive intake
Intoxication with salt-substitute, potassium-containing dietary supplements, or potassium chloride (KCl) infusion. Note that for a person with normal kidney function and nothing interfering with normal elimination (see above), hyperkalemia by potassium intoxication would be seen only with large infusions of KCl or oral doses of several hundred millequivalents of KCl.
[1]
Lethal injection
Hyperkalemia is intentionally brought about in an execution by lethal injection , with potassium chloride being the third and last of the three drugs administered to cause death.
Pseudohyperkalemia
Pseudohyperkalemia is a rise in the amount of potassium that occurs due to excessive leakage of potassium from cells, during or after blood is drawn. It is a laboratory artifact rather than a biological abnormality and can be misleading to caregivers.
[2]
Pseudohyperkalemia is typically caused by hemolysis during venipuncture (by either excessive vacuum of the blood draw or by a collection needle that is of too fine a gauge); excessive tourniquet time or fist clenching during phlebotomy (which presumably leads to efflux of potassium from the muscle cells into the bloodstream);
[3]
or by a delay in the processing of the blood specimen. It can also occur in specimens from patients with
10
abnormally high numbers of platelets (>1,000,000/mm³), leukocytes (> 100 000/mm³), or erythrocytes (hematocrit > 55%). People with "leakier" cell membranes have been found, whose blood must be separated immediately to avoid pseudohyperkalemia.
[4]
Pathophysiology
Potassium is the most abundant intracellular cation . It is critically important for many physiologic processes, including maintenance of cellular membrane potential , homeostasis of cell volume, and transmission of action potentials in nerve cells . Its main dietary sources are vegetables ( tomato and potato ), fruits ( orange and banana ) and meat .
Elimination is through the gastrointestinal tract and the kidney .
The renal elimination of potassium is passive (through the glomeruli ), and resorption is active in the proximal tubule and the ascending limb of the loop of Henle . There is active excretion of potassium in the distal tubule and the collecting duct ; both are controlled by aldosterone .
Hyperkalemia develops when there is excessive production (oral intake, tissue breakdown) or ineffective elimination of potassium. Ineffective elimination can be hormonal (in aldosterone deficiency) or due to causes in the renal parenchyma that impair excretion.
Increased extracellular potassium levels result in depolarization of the membrane potentials of cells. This depolarization opens some voltage-gated sodium channels , but not enough to generate an action potential. After a short while, the open sodium channels inactivate and become refractory , increasing the threshold to generate an action potential.
This leads to the impairment of neuromuscular, cardiac , and gastrointestinal organ systems. Of most concern is the impairment of cardiac conduction which can result in ventricular fibrillation or asystole .
During extreme exercise, potassium is released from active muscle and the serum potassium rises to a point that would be dangerous at rest. For unclear reasons, it appears as if the high levels of adrenaline and noradrenaline have a protective effect on the cardiac electrophysiology.
Patients with the rare hereditary condition of hyperkalemic periodic paralysis appear to have a heightened sensitivity of muscular symptoms that are associated with transient elevation of potassium levels. Episodes of muscle weakness and spasms can be precipitated by exercise or fasting in these subjects.
ECG findings
With mild to moderate hyperkalemia, there is reduction of the size of the P wave and development of peaked T waves . Severe hyperkalemia results in a widening of the QRS complex , and the EKG complex can evolve to a sinusoidal shape. There appears to be a
11
direct effect of elevated potassium on some of the potassium channels that increases their activity and speeds membrane repolarization. Also, (as noted above ), hyperkalemia causes an overall membrane depolarization that inactivates many sodium channels. The faster repolarization of the cardiac action potential causes the tenting of the T waves, and the inactivation of sodium channels causes a sluggish conduction of the electrical wave around the heart, which leads to smaller P waves and widening of the QRS complex.
Treatment
Acute : When arrhythmias occur, or when potassium levels exceed 6.5 mmol/l, emergency lowering of potassium levels is mandated. Several agents are used to lower K levels. Choice depends on the degree and cause of the hyperkalemia, and other aspects of the patient's condition.
Calcium supplementation (calcium gluconate 10% (10ml), preferably through a central venous catheter as the calcium may cause phlebitis ) does not lower potassium but decreases myocardial excitability, protecting against life threatening arrhythmias .
Insulin (e.g. intravenous injection of 10-15u of regular insulin {along with 50ml of 50% dextrose to prevent hypoglycemia}) will lead to a shift of potassium ions
into cells, secondary to increased activity of the sodium-potassium ATPase .
Bicarbonate therapy (e.g. 1 ampule (45mEq) infused over 5 minutes) is effective in cases of metabolic acidosis. The bicarbonate ion will stimulate an exchange of cellular H
+
for Na
+
, thus leading to stimulation of the sodium-potassium ATPase .
Salbutamol (albuterol, Ventolin) is a β
2
-selective catecholamine that is administered by nebulizer (e.g. 10–20 mg). This drug promotes movement of K into cells, lowering the blood levels.
Refractory or severe cases may need dialysis to remove the potassium from the circulation.
Prevention :
Preventing recurrence of hyperkalemia typically involves reduction of dietary potassium, removal of an offending medication, and/or the addition of a diuretic
(such as furosemide (Lasix) or hydrochlorothiazide ).
Polystyrene sulfonate (Calcium Resonium, Kayexalate) is a binding resin that binds K within the intestine and removes it from the body by defecation. Calcium
Resonium (15g three times a day in water) can be given by mouth. Kayexelate
(30g) can be given by mouth or as an enema . In both cases, the resin absorbs K within the intestine and carries it out of the body by defecation . This medication may cause diarrhea.
12
Causes
Hypokalemia can result from one or more of the following medical conditions:
Inadequate potassium intake
Perhaps the most obvious cause is insufficient consumption of potassium (that is, a low-potassium diet). However, without excessive potassium loss from the body, this is a rare cause of hypokalemia.
Gastrointestinal/integument loss
A more common cause is excessive loss of potassium, often associated with heavy fluid losses that "flush" potassium out of the body. Typically, this is a consequence of diarrhea , excessive perspiration , or losses associated with surgical procedures. Vomiting can also cause hypokalemia, although not much potassium is lost from the vomitus. Rather, there are heavy urinary losses of K + in the setting of postemetic bicarbonaturia that force urinary potassium excretion (see
Alkalosis below).
Urinary loss
Certain medications can cause excess potassium loss in the urine. Diuretics , including thiazide diuretics (e.g. hydrochlorothiazide ) and loop diuretics (e.g. furosemide ) are a common cause of hypokalemia. Other medications such as the antifungal, amphotericin B , or the cancer drug, cisplatin , can also cause long-term hypokalemia.
A special case of potassium loss occurs with diabetic ketoacidosis . In addition to urinary losses from polyuria and volume contraction, there is also obligate loss of potassium from kidney tubules as a cationic partner to the negatively charged ketone
, β-hydroxybutyrate.
Hypomagnesemia can cause hypokalemia. Magnesium is required for adequate processing of potassium. This may become evident when hypokalemia persists despite potassium supplementation. Other electrolyte abnormalities may also be present.
Alkalosis can cause transient hypokalemia by two mechanisms. First, the alkalosis causes a shift of potassium from the plasma and interstitial fluids into cells; perhaps mediated by stimulation of Na
+
-H
+
exchange and a subsequent activation of Na + /K + -ATPase activity.
[3] Second, an acute rise of plasma HCO
3
concentration (caused by vomiting, for example) will exceed the capacity of the renal proximal tubule to reabsorb this anion , and potassium will be excreted as an obligate cation partner to the bicarbonate.
13
Disease states that lead to abnormally high aldosterone levels can cause hypertension and excessive urinary losses of potassium. These include renal artery stenosis and tumors (generally non-malignant) of the adrenal glands.
Hypertension and hypokalemia can also be seen with a deficiency of the 11-betahydroxysteroid dehydrogenase type 2 enzyme which allows cortisols to stimulate aldosterone receptors. This deficiency -- known as apparent mineralocorticoid excess syndrome -- can either be congenital or caused by consumption of glycyrrhizin , which is contained in extract of licorice, sometimes found in herbal supplements , candies and chewing tobacco.
Distribution away from ECF
In addition to alkalosis, other factors can cause some shifting of potassium into cells -- presumably by stimulation of the Na-K-ATPase -- such as insulin, epinephrine and other beta agonists , and xanthines (eg. Theophylline ).
[5]
Rare hereditary defects of muscular ion channels and transporters that cause hypokalemic periodic paralysis can precipitate occasional attacks of severe hypokalemia and muscle weakness. These defects cause a heightened sensitivity to the normal changes in potassium produced by catechols and/or insulin and/or thyroid hormone , which lead to movement of potassium from the extracellular fluid into the muscle cells.
Potassium is essential for many body functions, including muscle and nerve activity. The electrochemical gradient of potassium between the intracellular and extracellular space is essential for nerve function; in particular, potassium is needed to repolarize the cell membrane to a resting state after an action potential has passed. Decreased potassium levels in the extracellular space will cause hyperpolarization of the resting membrane potential. This hyperpolarization is caused by the effect of the altered potassium gradient on resting membrane potential as defined by the Goldman equation . As a result, a greater than normal stimulus is required for depolarization of the membrane in order to initiate an action potential.
In certain conditions, this will make cells less excitable. However, in the heart, it causes myocytes to become hyperexcitable. Lower membrane potentials in the atrium may cause arrhythmias because of more complete recovery from sodium-channel inactivation, making the triggering of an action potential more likely. In addition, the reduced extracellular potassium (paradoxically) inhibits the activity of the I
Kr
potassium current
[10] and delays ventricular repolarization. This delayed repolarization may promote reentrant arrythmias .
Treatment
The most important treatment in severe hypokalemia is addressing the cause, such as improving the diet, treating diarrhea or stopping an offending medication. Patients
14
without a significant source of potassium loss and who show no symptoms of hypokalemia may not require treatment.
Mild hypokalemia (>3.0 mEq/L) may be treated with oral potassium chloride supplements (Klor-Con, Sando-K, Slow-K). As this is often part of a poor nutritional intake, potassium-containing foods may be recommended, such as leafy green vegetables, tomatoes , citrus fruits, oranges or bananas . Both dietary and pharmaceutical supplements are used for people taking diuretic medications
Severe hypokalemia (<3.0 mEq/L) may require intravenous supplementation. Typically, saline is used, with 20-40 mEq KCl per liter over 3-4 hours. Giving intravenous potassium at faster rates (20-25 mEq/hr) may predispose to ventricular tachycardias and requires intensive monitoring. A generally safe rate is 10 mEq/hr.
Difficult or resistant cases of hypokalemia may be amenable to a potassium-sparing diuretic such as amiloride , triamterene , or spironolactone . In contrast to the more commonly used diuretics like hydrochlorothiazide and furosemide , these potassiumsparing diuretics actually reduce the kidney's excretion of potassium.
When replacing potassium intravenously, infusion via central line is encouraged to avoid the frequent occurrence of a burning sensation at the site of a peripheral IV, or the rare occurrence of damage to the vein. When peripheral infusions are necessary, the burning can be reduced by diluting the potassium in larger amounts of IV fluid, or mixing 3 ml of
1% lidocaine to each 10 meq of kcl per 50 ml of IV fluid. The practice of adding lidocaine, however, raises the likelihood of serious medical errors.
[12]
Hyponatremia
Hyponatremia ( British : hyponatraemia ) is an electrolyte disturbance (a disturbance of the salts in the blood) in which the sodium ( Natrium in Latin ) concentration in the plasma is lower than normal ( hypo in Greek; in this case, below 135 mmol/L).
[1]
. The large majority of cases of hyponatremia occurring in adults result from an excess amount or effect of the water retaining hormone known as Antidiuretic Hormone commonly abbreviated as ADH.
Hyponatremia is most often a complication of other medical illnesses in which either fluids rich in sodium are lost (for example because of diarrhea or vomiting ), or excess water accumulates in the body at a higher rate than it can be excreted (for example in polydipsia (rarely) or syndrome of inappropriate antidiuretic hormone , SIADH).
Regarding sodium loss as a cause of hyponatremia, it is important to note that such losses promote hyponatremia only in an indirect manner. In particular, hyponatremia occurring in association with sodium loss does not reflect inadequate sodium availability as a result of the losses. Rather, the sodium loss leads to a state of volume depletion , with volume depletion serving as a signal for the release of ADH. As a result of ADH-stimulated water retention, blood sodium becomes diluted and hyponatremia results.
15
There may also be spurious hyponatremia ( pseudohyponatremia or factitious hyponatremia ) if other substances expand the serum and dilute the sodium (for example, high blood sugar ( hyperglycemia ) or if a blood constituent leads to the creation of a sodium-free phase in the blood thereby causing the blood plasma volume to be overestimated (e.g. extreme hypertriglyceridemia ).
Hyponatremia can also affect athletes who consume too much fluid during endurance events,
[2]
people who fast on juice or water for extended periods and people whose dietary sodium intake is chronically insufficient.
The diagnosis of hyponatremia relies mainly on the medical history , clinical examination and blood and urine tests . Treatment can be directed at the cause (for example, corticosteroids in Addison's disease ) or involve restriction of water intake, intravenous saline or drugs like diuretics , demeclocycline , urea or vaptans ( antidiuretic hormone receptor antagonists ). Correcting the salt and fluid balance needs to occur in a controlled fashion, as too rapid correction can lead to severe complications such as heart failure or a sometimes irreversible brain lesion known as central pontine myelinolysis .
Symptoms
Patients with low-level, chronic water intoxication are often asymptomatic, but may have symptoms related to the underlying cause.
Severe hyponatremia in acute or chronic form may cause osmotic shift of water from the plasma into the brain cells . Typical symptoms include nausea , vomiting , headache and malaise . As the hyponatremia worsens, confusion, diminished reflexes , convulsions , stupor or coma may occur. Since nausea is, itself, a stimulus for the release of ADH , which promotes the retention of water, a positive feedback loop may be created and the potential for a vicious cycle of hyponatremia and its symptoms exists.
A feedback loop can also be created by severe thirst, which is a symptom of some hyponatremic individuals.
[3]
When these people consume large quantities of water without an adequate increase in sodium, the hyponatremic condition worsens.
Causes
One approach to determining causes of hyponatremia
An abnormally low plasma sodium level is best considered in conjunction with the person's plasma osmolality and extracellular fluid volume status. Indeed, correct ascertainment of volume status, as well as determination of the presence or absence of edema , are both critical in establishing the cause of hyponatremia. As described above, a state of volume depletion leads to increased blood levels of ADH and thus water
16
retention. The greater the amount of water that is retained, the more the blood sodium will become diluted to cause worsening degrees of hyponatremia. The presence of edema indicates that blood volume has been lost insofar as fluid from the blood has shifted out into the peripheral tissues to cause the edema. In other words, edema is usually reflecting a state of blood volume depletion. As a result, edematous states are also associated with increased blood levels of ADH, water retention, and hyponatremia. In all cases of volume depletion-associated hyponatremia, it is important to note that retention of water per se , such as that promoted by ADH, does not correct the volume depleted state.
In addition to volume depletion, there are other causes of increased ADH levels (and ultimately, therefore, of hyponatremia). These include nausea, pain, and opiate drugs such as codeine and morphine. Such factors often play a role in the hyponatremia that is frequently seen in hospitalized patients.
Type
Serum osmolality
(mOsm/kg)
Description
Hypotonic hyponatremia
Isotonic hyponatremia between 280 and 295
Hypertonic hyponatremia
< 280
> 295
When the plasma osmolality is low, the extracellular fluid volume status may be in one of three states: low volume , normal volume , or high volume .
Certain conditions that interfere with laboratory tests of serum sodium concentration (such as extraordinarily high blood levels of lipid or protein ) may lead to an erroneously low measurement of sodium.
[4]
This is called pseudohyponatremia .
Hypertonic hyponatremia can be associated with shifts of fluid due to osmotic pressure.
[4]
Although accounting for only a small minority of cases, hyponatremia due to excessive water intake does occur. This form of hyponatremia is not due to ADH action. Rather, in such instances, the amount of water ingested has exceeded the kidney's ability to excrete it. This typically occurs when the kidney lacks sufficient solute to accompany the water.
In other words, the kidney cannot excrete pure water. Water must be accompanied by a solute such as urea in order to become urine. Hyponatremia arising due to a lack of solute in the kidney can occur if there has been grossly inadequate intake of nutrition (and thus inadequate urea production) at the same time as there has been excessive water intake
(e.g. "beer potomania"). It can also occur even in normally nourished individuals when huge quantities (> 12 liters/day) of water have been ingested. Such high volumes of water require a solute accompaniment which exceeds the availability of solute even to the kidneys of a well-nourished individual.
17
Treatment
The treatment of hyponatremia usually depends on the underlying cause. If a person has few symptoms, little treatment other than water restriction may be required. In the setting of volume depletion , intravenous administration of normal saline may be effective.
Over aggressive correction of hyponatremia may lead to a syndrome of central pontine myelinolysis . Thus, correction of serum sodium should not exceed 12 mEq/L per 24 hours nor 18 mEq/L per 48h.
Seizures associated with hyponatremia are typically treated with a 100 mL bolus of 3 % hypertonic saline.
[5]
Hypernatremia
Hypernatremia or hypernatraemia is an electrolyte disturbance that is defined by an elevated sodium level in the blood.
Hypernatremia is generally not caused by an excess of sodium, but rather by a relative deficit of free water in the body. For this reason, hypernatremia is often synonymous with the less precise term, dehydration.
Water is lost from the body in a variety of ways, including perspiration , insensible losses from breathing, and in the feces and urine . If the amount of water ingested consistently falls below the amount of water lost, the serum sodium level will begin to rise, leading to hypernatremia. Rarely, hypernatremia can result from massive salt ingestion, such as may occur from drinking seawater .
Ordinarily, even a small rise in the serum sodium concentration above the normal range results in a strong sensation of thirst , an increase in free water intake, and correction of the abnormality. Therefore, hypernatremia most often occurs in people such as infants , those with impaired mental status , or the elderly, who may have an intact thirst mechanism but are unable to ask for or obtain water.
18
Common causes of hypernatremia include:
Hypovolemic o Inadequate intake of water, typically in elderly or otherwise disabled patients who are unable to take in water as their thirst dictates. This is the o most common cause of hypernatremia.
Excessive losses of water from the urinary tract, which may be caused by glycosuria , or other osmotic diuretics. o Water losses associated with extreme sweating. o Severe watery diarrhea
Euvolemic o Excessive excretion of water from the kidneys caused by diabetes insipidus , which involves either inadequate production of the hormone, vasopressin , from the pituitary gland or impaired responsiveness of the kidneys to vasopressin.
Hypervolemic o Intake of a hypertonic fluid (a fluid with a higher concentration of solutes than the remainder of the body). This is relatively uncommon, though it can occur after a vigorous resuscitation where a patient receives a large volume of a concentrated sodium bicarbonate solution. Ingesting seawater o also causes hypernatremia because seawater is hypertonic.
Mineralcorticoid excess due to a disease state such as Conn's syndrome or
Cushing's Disease
Symptoms
Clinical manifestations of hypernatremia can be subtle, consisting of lethargy , weakness, irritability, and edema . With more severe elevations of the sodium level, seizures and coma may occur.
Severe symptoms are usually due to acute elevation of the plasma sodium concentration to above 158 mEq/L (normal is typically about 135-145 mEq/L). Values above 180 mEq/L are associated with a high mortality rate, particularly in adults. However such high levels of sodium rarely occur without severe coexisting medical conditions.
Treatment
The cornerstone of treatment is administration of free water to correct the relative water deficit. Water can be replaced orally or intravenously . However, overly rapid correction of hypernatremia is potentially very dangerous. The body (in particular the brain ) adapts to the higher sodium concentration. Rapidly lowering the sodium concentration with free water, once this adaptation has occurred, causes water to flow into brain cells and causes them to swell. This can lead to cerebral edema , potentially resulting in seizures, permanent brain damage , or death. Therefore, significant hypernatremia should be treated carefully by a physician or other medical professional with experience in treatment of electrolyte imbalances .
19
Dehydration
.
Dehydration ( hypohydration ) is defined as excessive loss of body fluid .
There are three main types of dehydration; hypotonic (primarily a loss of electrolytes, sodium in particular), hypertonic (primarily a loss of water), and isotonic (equal loss of water and electrolytes).
In humans, the most commonly seen type of dehydration by far is isotonic (isonatraemic) dehydration which effectively equates with hypovolaemia (described below), but distinction of isotonic from hypotonic or hypertonic dehydration may be important when treating people who become dehydrated. It is important to understand (physiologically speaking) that dehydration, despite the name, does not simply mean loss of water, as water and solutes (mainly sodium) are usually lost in roughly equal quantities to how they exist in blood plasma .
Medical causes of dehydration
In humans, dehydration can be caused by a wide range of diseases and states that impair water homeostasis in the body. These include:
External or stress -related causes o Prolonged physical activity with sweating without consuming adequate water, especially in a hot and/or dry environment o Prolonged exposure to dry air, e.g. in high-flying airplanes (5–12% relative humidity)
Blood loss or hypotension due to physical trauma o o o o o o o o o
Diarrhea
Shock
Burns
Use of
Hyperthermia
(hypovolemic)
Vomiting
Lacrimation methamphetamine ,
Excessive consumption of amphetamine , caffeine alcoholic beverages
and other stimulants
Infectious diseases o o o o
Cholera
Gastroenteritis
Shigellosis
Yellow fever
20
Malnutrition o Electrolyte disturbance
Hypernatremia (also caused by dehydration)
Hyponatremia , especially from restricted salt diets o o
Fasting
Recent rapid weight loss may reflect progressive depletion of fluid volume o o
(the loss of 1 L of fluid results in a weight loss of 1 kg
Patient refusal of nutrition and hydration
Inability to swallow (obstruction of the oesophagus)
Other causes of obligate water loss o Severe
hyperglycemia
Glycosuria
Uremia
, especially in Diabetes mellitus
Symptoms and prognosis
Symptoms may include headaches similar to what is experienced during a hangover , muscle cramps, a sudden episode of visual snow , decreased blood pressure ( hypotension ), and dizziness or fainting when standing up due to orthostatic hypotension . Untreated dehydration generally results in delirium , unconsciousness , swelling of the tongue and in extreme cases death .
Dehydration symptoms generally become noticeable after 2% of one's normal water volume has been lost. Initially, one experiences thirst and discomfort, possibly along with loss of appetite and dry skin . This can be followed by constipation .
Symptoms of mild dehydration include thirst , decreased urine volume, abnormally dark urine, unexplained tiredness, irritability, lack of tears when crying, headache , dry mouth, dizziness when standing due to orthostatic hypotension , and in some cases can cause insomnia. Blood tests may show hyperalbuminemia .
In moderate to severe dehydration, there may be no urine output at all. Other symptoms in these states include lethargy or extreme sleepiness, seizures , sunken fontanel (soft spot) in infants, fainting , and sunken eyes.
The symptoms become increasingly severe with greater water loss. One's heart and respiration rates begin to increase to compensate for decreased plasma volume and blood pressure , while body temperature may rise because of decreased sweating. Around 5% to
6% water loss, one may become groggy or sleepy, experience headaches or nausea , and may feel tingling in one's limbs ( paresthesia ). With 10% to 15% fluid loss, muscles may become spastic, skin may shrivel and wrinkle (decreased skin turgor), vision may dim, urination will be greatly reduced and may become painful, and delirium may begin.
Losses greater than 15% are usually fatal.
21
In people over age 50, the body’s thirst sensation diminishes and continues diminishing with age. Many senior citizens suffer symptoms of dehydration. Dehydration along with hyperthermia results in seniors dying during extreme hot weather.
Diseases of the gastrointestinal tract can lead to dehydration in various ways. Often, dehydration becomes the major problem in an otherwise self-limited illness. Fluid loss may even be severe enough to become life-threatening.
Numerous studies have shown that for terminally ill patients who choose to die, deaths by dehydration are generally peaceful, and not associated with suffering, when supplemented with adequate pain medication.
Treatment
Nurses encourage a patient to drink an Oral Rehydration Solution to improve dehydration he acquired from cholera .
The treatment for minor dehydration often considered the most effective is drinking water and stopping fluid loss. Plain water restores only the volume of the blood plasma, inhibiting the thirst mechanism before solute levels can be replenished.
In more severe cases, correction of a dehydrated state is accomplished by the replenishment of necessary water and electrolytes ( rehydration , through oral rehydration therapy or intravenous therapy ). Even in the case of serious lack of fresh water (e.g. at sea or in a desert), drinking seawater or urine does not help, nor does the consumption of alcohol . It is often thought that the sudden influx of salt into the body from seawater will cause the cells to dehydrate and the kidneys to overload and shut down but it has been calculated that an average adult can drink up to 0.1 litres of seawater per day before the kidneys start to fail
[ citation needed ]
For severe cases of dehydration where fainting , unconsciousness , or other severely inhibiting symptom is present (the patient is incapable of standing or thinking clearly), emergency attention is required. Fluids containing a proper balance of replacement electrolytes are given orally or intravenously with continuing assessment of electrolyte status; complete resolution is the norm in all but the most extreme cases.
Avoiding dehydration
Dehydration is best avoided by drinking sufficient water . The greater the amount of water lost through perspiration, the more water must be consumed to replace it and avoid dehydration. Since the body cannot tolerate large deficits or excesses in total body water, consumption of water must be roughly concurrent with the loss (in other words, if one is perspiring, one should also be drinking some water frequently).
22
For routine activities in which a person is not perspiring to any large degree, drinking when one is thirsty is sufficient to maintain hydration. However, during exercise, relying on thirst alone may be insufficient to prevent dehydration from occurring. This is particularly true in hot environments, or for those older than 65. For an exercise session, an accurate determination of how much fluid is necessary to consume during the workout can be made by performing appropriate weight measurements before and after a typical exercise session, to determine how much fluid is lost during the workout.
[14][15][16][17][18]
Drinking water beyond the needs of the body entails little risk when done in moderation, since the kidneys will efficiently remove any excess water through the urine with a large margin of safety.
A person's body, during an average day in a temperate climate such as the United
Kingdom , loses approximately 2.5 litres of water.
[ citation needed ]
This can be through the lungs as water vapor , through the skin as sweat , or through the kidneys as urine . Some water (a less significant amount, in the absence of diarrhea ) is also lost through the bowels . In warm or humid weather or during heavy exertion, however, the water loss can increase by an order of magnitude or more through perspiration; all of which must be promptly replaced. In extreme cases, the losses may be great enough to exceed the body's ability to absorb water from the gastrointestinal tract; in these cases, it is not possible to drink enough water to stay hydrated, and the only way to avoid dehydration is to either pre-hydrate, or find ways to reduce perspiration (through rest, a move to a cooler environment, etc.)
A useful rule of thumb for avoiding dehydration in hot or humid environments or during strenuous activity involves monitoring the frequency and character of urination. If one develops a full bladder at least every 3-5 hours and the urine is only lightly colored or colorless, chances are that dehydration is not occurring; if urine is deeply colored, or urination occurs only after many hours or not at all, water intake may not be adequate to maintain proper hydration.
When large amounts of water are being lost through perspiration and concurrently replaced by drinking, maintaining proper electrolyte balance becomes an issue. Drinking fluids that are hypertonic or hypotonic with respect to perspiration may have grave consequences ( hyponatremia or hypernatremia , principally) as the total volume of water turnover increases.
If water is being lost through abnormal mechanisms such as vomiting or diarrhea , an imbalance can develop very quickly into a medical emergency.
During sports events such as marathons , water stops and water breaks are provided to avoid dehydration of athletes.
23