t7 determination of the isentropic exponent of air
advertisement
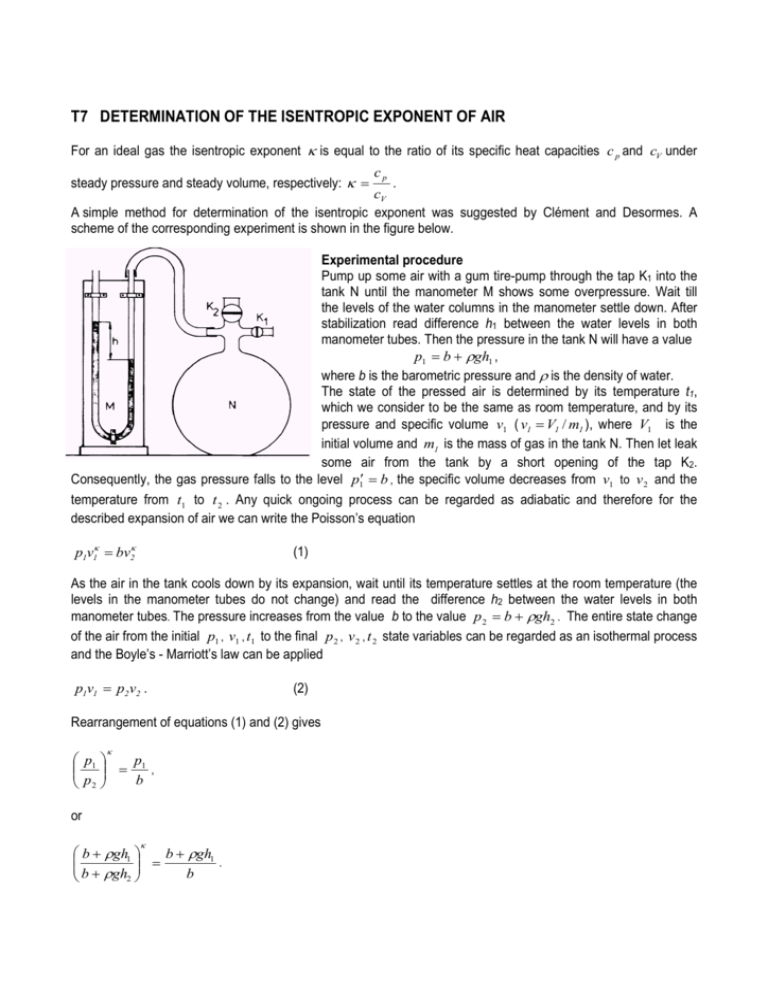
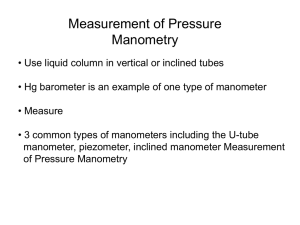
T7 DETERMINATION OF THE ISENTROPIC EXPONENT OF AIR For an ideal gas the isentropic exponent κ is equal to the ratio of its specific heat capacities c p and cV under steady pressure and steady volume, respectively: κ = cp . cV A simple method for determination of the isentropic exponent was suggested by Clément and Desormes. A scheme of the corresponding experiment is shown in the figure below. Experimental procedure Pump up some air with a gum tire-pump through the tap K1 into the tank N until the manometer M shows some overpressure. Wait till the levels of the water columns in the manometer settle down. After stabilization read difference h1 between the water levels in both manometer tubes. Then the pressure in the tank N will have a value p1 = b + ρgh1 , where b is the barometric pressure and ρ is the density of water. The state of the pressed air is determined by its temperature t1, which we consider to be the same as room temperature, and by its pressure and specific volume v1 ( v1 = V1 / m1 ), where V1 is the initial volume and m1 is the mass of gas in the tank N. Then let leak some air from the tank by a short opening of the tap K2. Consequently, the gas pressure falls to the level p1′ = b , the specific volume decreases from v1 to v 2 and the temperature from t1 to t 2 . Any quick ongoing process can be regarded as adiabatic and therefore for the described expansion of air we can write the Poisson’s equation p1v1κ = bv2κ (1) As the air in the tank cools down by its expansion, wait until its temperature settles at the room temperature (the levels in the manometer tubes do not change) and read the difference h2 between the water levels in both manometer tubes. The pressure increases from the value b to the value p 2 = b + ρgh2 . The entire state change of the air from the initial p1 , v1 , t1 to the final p 2 , v 2 , t 2 state variables can be regarded as an isothermal process and the Boyle’s - Marriott’s law can be applied p1v1 = p2v2 . (2) Rearrangement of equations (1) and (2) gives κ p1 p = 1 , b p2 or κ b + ρgh1 b + ρgh1 = . b b + ρgh2 Then the Poisson’s constant (isentropic exponent) κ is equal to b + ρgh1 log b . κ= b + ρgh1 log + b ρ gh 2 If ρgh1 b << 1 , ρgh2 b (3) << 1 , the expression for κ can be simplified using approximation for small values of x : log(1 + x ) ≅ x . ρgh1 b + ρgh1 ρgh1 log log1 + h1 b b = b ≅ ≅ κ= b + ρgh1 ρgh1 − ρgh2 ρgh1 − ρgh2 h1 − h2 log1 + log b + ρgh2 b + ρgh2 b + ρgh2 and hence κ can be expressed with sufficient accuracy by the expression κ′ = h1 . h1 − h2 (4) Record the values of barometric pressure b at the beginning and the end of the experiment and calculate the average value of both values. Repeat the experiment 10 times at different values of h1 and different speed values of the tap K2 opening. Record the values h1, h2 in the table. Calculate κ values using the exact (3) and the approximate (4) formulas and record them in the table. For κ and κ ′ calculate the percent of difference with respect to the table value for air κ tab and record the results in the table. Notes 1. 1 Torr = 133.3 Pa. 2. The values of water density ρ = 1000 kgm −3 . 3. The table value of the isentropic exponent (Poisson constant) for air is κ tab = 1.405 . 4. When inflating air into the tank, it is necessary to pay attention not to spill the water since one tube of manometer is open.