339400_0_data_set_3011678_lmjffm
advertisement

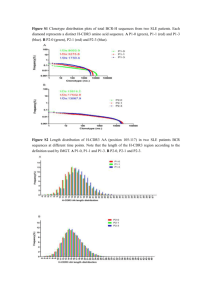
Electronic Supplementary Information Tailoring oxidation degrees of graphene oxide by simple chemical reactions Gongkai Wang, a,b Xiang Sun,b Changsheng Liua and Jie Lian*b Experimental Section Synthesis of GO: All chemicals used for GO synthesis are from Sigma-Aldrich. Graphite powders (Sigma-Aldrich, cat# 282863, ~20μm) were oxidized by four different procedures, which were briefly summarized in Table 1, yielding four GO samples with different oxidation degrees, denoted as No-1, No-2, No-3 and No-4, respectively. Table 1-Summary of GO Samples and Synthesis Conditions Sample Synthesis Conditions No-1 Soak graphite in concentrated H2SO4 for 12 h, KMnO4 added, stirred for 3 h at room temperature No-2 Soak graphite in concentrated H2SO4 for 12h, KMnO4 added, stirred for 1 h at room temperature, then heated at 85 °C for 1 h No-3 Hummers Method No-4 Graphite, sonicated in H2SO4/HNO3(3:1) for 4 h In detail, for the sample GO No-1, graphite (150 mg) powders were dispersed in concentrated H2SO4 (30 ml) and stirred for 12 h at room temperature. KMnO4 (750 mg) was then added slowly into the solution, and the mixture was stirred again for 3 h at room temperature. Then DI water (400 ml) containing 5 ml H2O2 (3 wt%) was added into the solution in an ice bath followed by vacuum filtration through PVDF membranes (Millipore membranes, GVWP04700, 0.22μm pore size). The solid was removed and subjected to multistep washing and filtration twice: first, the solid was dispersed in 150 ml DI water followed by stirring for 30 min and vacuum filtration; then the solid was washed with 20 vol% HCl (150 ml) followed by bath sonication for 30 min and vacuum filtration; the solid was then removed by vacuum filtration after stirring in ethanol (150 ml) for 30 min. The products were finally washed with DI water (150 ml) and dried in vacuum at 40 °C for 12 h. For the GO No-2 sample, graphite powders (150 mg) were dispersed in concentrated H2SO4 (30 ml) and stirred for 12 h at room temperature. KMnO4 (750 mg) was added slowly to the solution, the mixture was stirred for 1 h at room temperature followed by heating the solution to 85 °C and keeping there for 1h. The washing procedures are the same as that mentioned above in GO No-1. The final product was dried in vacuum at 40 °C for 12 h. The GO No-3 sample was synthesized by the Hummers method.1 Briefly, graphite powders (5 g) were added to the mixture of concentrated H2SO4 (115 ml) and NaNO3 (2.5 g) and stirred in an ice bath (0 °C). KMnO4 (15 g) was added carefully to avoid the temperature of the solution exceeding 20 °C. The solution was then maintained for 30 min at 35 °C followed by an addition of DI water (230 ml) slowly. The temperature of the reaction was turned to 98 °C and the solution was kept for 15 min. After that additional DI water (355 ml) containing H2O2 (3 wt%, 5 ml) was added. The final yellow brown powders were obtained by following the same washing procedures as mentioned above in the sample GO No-1. The final products were dried in vacuum at 40 °C for 12 h. For the sample No-4, graphite powders (150 mg) were suspended in a mixture of concentrated H2SO4/HNO3 (3:1) (30 ml) and sonicated in a bath cleaner for 4 h. The resultant solution was diluted with DI water (400 ml) containing H2O2 (3 wt%, 5 ml) followed by vacuum filtration through PVDF membranes. Washing procedures are the same to that of the sample GO No-1. The final products were dried in vacuum at 40 °C for 12 h. The sample No-4 by simply acid treatment is a control sample for comparison purpose. Material Characterization Upon materials synthesis, a wide range of characterization approaches were employed to evaluate the structural properties and oxidation degrees of the as-synthesized GO including X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), gravimetric analysis (TGA), Raman and UV-vis-IR spectrophotometers. XRD was performed on a PANalytical X-ray diffraction system with a source wavelength of 1.542 Å at room temperature, and XPS was carried out using a PHI 5000 Versa Probe system. Thermal gravimetric analysis (TGA) was determined by a TA Instruments TGA-Q50. The heating rate was 20 °C min-1 from room temperature to 800 °C in a nitrogen atmosphere. Raman spectroscopy was recorded with a Jobin-Yvon HR300 Raman spectrometer equipped with a 532 nm green laser source, and the UV-vis spectra was acquired with a VARIAN 6000i UV-vis-IR spectrophotometer. Fig. S1 TGA curves of the different GO samples at the heating rate of 20 °C min-1 from room temperature to 800 °C in N2 atmosphere. Fig. S2 Raman spectra of GO samples. For Raman spectra of the GO samples shown in Fig. S2, two prominent peaks can be observed, corresponding to D band (~1353 cm-1) and G band (~1592 cm-1). The G band was induced by the in-plane optical vibration (degenerate zone center E2g mode), and the D band existed in the defective graphene due to the first-order zone boundary phonons. 2 The red-shift tendency of G band from the GO No-1 sample to the No-4 sample can be observed, indicating that the concentration of defects on the graphene plane decreased. Another band (D’ band) located at 1620 cm-1 gradually merges with G band with increasing defects. 3 In addition, the peak of G band becomes prominent from the No-1 to No-4 GO samples, suggesting the augmentation in size of the in-plane sp2 domains due to the reduction of the number of defective sites. 4 As in the case of the D band, the intensity is higher than the corresponding G bands from the GO No-1 to No-3 samples, illustrating that all of them have high defect densities. The D and G band intensity ratio can be used to evaluate the disorder degree of materials. As calculated, the decrease tendency of the D/G ratio from the samples GO NO-1 to No-3 and the sample No-4 is 1.08, 1.05, 1.05, 0.32, respectively, suggesting an increase in the average size of the sp2 domains. It is concluded that the hexagonal structure of the GO No-1 was disrupted significantly upon oxidation processes, resulting in a high defect density and the highest oxidation degree among all of these samples. In contrast, the sample No-4 was only slightly oxidized. Reference 1 2 W. S. Hummers and R. E. Offeman, Journal of the American Chemical Society 80 , 1339 (1958). Y. W. Zhu, S. Murali, W. W. Cai, X. S. Li, J. W. Suk, J. R. Potts, and R. S. Ruoff, Advanced Materials 22 , 3906 (2010). 3 K. N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prud'homme, I. A. Aksay, and R. Car, Nano Letters 8 , 36 (2008). 4 S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen, and R. S. Ruoff, Carbon 45, 1558 (2007).