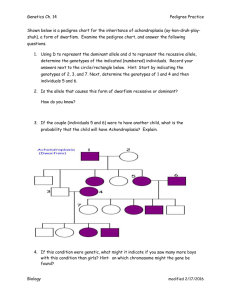
observed genotypes
advertisement

The interleukin-8 (-251A/T) polymorphism is associated with increased risk for oral squamous cell carcinoma E.Vairaktarisa, C.Yapijakisa,b,*, Z. Serefogloua,b, S. Derkaa, S. Vassilioua, E. Nkenkec, A. Vylliotisa, J. Wiltfangd, D. Avgoustidisa, E. Critselisa, E Patsourise, FW. Neukamc a Department of Maxillofacial Surgery, University of Athens Medical School, Vas. Sofias 93 & Dim. Soutsou 1, GR-11521 Athens, Greece b Department of Molecular Biology and Genetics, "Bioerevna" Research Center, Marni 4, GR- 10433 Athens, Greece c Department of Maxillofacial Surgery, Universität Erlangen, Klinik und Poliklinik für Mund-, Kiefer-, Gesischtschirurgie, Glueckstrasse 11, Erlagen D-91054, Nürnberg, Germany d Department of Maxillofacial Surgery, Universität Kiel, Klinik und Poliklinik für Mund-, Kiefer-, Gesischtschirurgie, Arnold Heller 16, D-24105 Kiel, Germany e Department of Pathology, University of Athens Medical School, Vas. Sofias 93 & Dim. Soutsou 1, GR-11521 Athens, Greece (*) Corresponding author: Christos Yapijakis, DMD,MS,PhD, Department of Molecular Biology and Genetics, "Bioerevna" Research Center, Marni 4, GR-10433 Athens, Greece Tel+30-210-8213444; Fax+30-210-8811243; Email cyapijakis_ua_gr@yahoo.com Financial Support: EPEAEK “Pythagoras” grant 70/3/7391 of the Greek Secretariat of Research and Technology 1 Abstract Aims: In light of recently found contribution of angiogenic and inflammation-related factors to malignancies, this study investigated the possible association of interleukin-8 gene (IL-8) to increased risk of oral cancer. Methods: The IL-8 (-251A/T) polymorphism, which influences IL-8 gene expression, was evaluated by restriction fragment length polymorphism analysis in DNA samples of 168 German and Greek patients with oral squamous cell carcinoma and 159 healthy controls of equivalent sex, ethnicity and age. Results: Significant increase of mutant (A-251) allele, which results in higher IL-8 gene expression, was observed in all patients in comparison to normal controls (P<0.001). The A/T heterozygotes had a two-fold greater risk (odds ratio 1.76, CI 1.11-2.79) for developing oral cancer compared to normal TT homozygotes. Furthermore, significantly increased values of mutant allele frequencies compared to controls were observed in all patients as well as in subgroups of patients with or without positive history of cancer (P<0.05 and P<0.001, respectively) and with or without positive history of thrombophilia (P<0.05 and P<0.001, respectively). Conclusions:In light to known observations of elevated plasma levels of IL-8 in several types of cancer including oral squamous cell carcinoma, the findings of this study suggest that the mutant allele of the (-251A/T) polymorphism may be a major contributing genetic factor to risk for oral cancer. Keywords: Interleukin-8; Oral cancer; Angiogenesis; Thrombophilia; Polymorphism 2 Introduction Oral squamous cell carcinoma is one of the most common malignancies worldwide. Environmental factors (such as smoking, alcohol) and genetic alterations in oncogenes and tumor suppressor genes modulate individual susceptibility to oral cancer1. Recently, factors related to angiogenesis and thrombosis have been implicated in increased risk for malignancy in the oral region2-6. One such factor known to be involved in thrombophilia, angiogenesis and some types of cancer is interleukin 8 (IL-8)7-9. IL-8 is a cytokine produced by monocytes, T lymphocytes, neutrophils, endothelial and epithelial cells, and fibroblasts10. It regulates humoral and immune responses through its inflammatory and procoagulant properties7,10. In addition, IL-8 has been involved in angiogenesis and neovascularization-dependent tumor growth8,11. The (-251A/T) polymorphism, which is found in the promoter region of the IL-8 gene, is the only one known to influence its expression12. The less common allele A results in increased levels of IL-8 and has been associated with increased risk for gastric and prostate cancer12-14. The mutant A allele frequency ranges between 18-25% in Europeans and Asians13-14. On the other hand, elevated levels of IL-8 protein have been observed in patients with ovarian and hepatocellular cancer, as well as in oral squamous carcinoma cell lines15-17. Interestingly, patients with metastatic melanoma who responded to chemotherapy showed a significant decrease in the serum level for IL-89. In light of the above, we investigated the possible correlation of the (-251A/T) IL-8 polymorphism with risk for oral cancer studying a cohort of patients with oral squamous cell carcinoma and healthy controls representing the general population. 3 Patients and Methods The individuals under study were 314 Greeks and Germans, recruited by the participating departments. They included 158 patients with squamous cell carcinoma in the oral cavity and 156 healthy blood donors of similar age, ethnicity and sex. The demographic characteristics of studied patients and controls are shown in Table 1. The patients, who were included in this study, had developed oral cancer and were operated recently or up to a decade ago. In addition to clinical presentation, a biopsy with pathological diagnosis of tumor stages I-IV and a family history regarding cancer and thrombophilia were available. Sixty patients (38%) had one or two first degree relatives with any type of cancer and their age range (41-83 years; 58.7±/10.2, mean age=58.7 years) did not differ significantly from the whole group of patients. Furthermore, thirty two patients (20.3%) had one or two first-degree with idiopathic thrombosis and an earlier age range (44-75 years; 58±/9.9, mean age=58 years) but again with no statistical difference compared to the whole group. Sixteen patients (10.1%) had a positive family history for both cancer and thrombophilia (48-74 years; 56.3±/8, mean age= 56.3 years). Most of the participants in the two groups worked in a low-risk environment (with the exception of one patient and three controls who worked in chemical factories). No data were available on controls regarding their family history or smoking and alcohol consumption habits. Blood samples were collected from patients and controls under study after informed consent. DNA was isolated from blood with the use of Nucleon TM kit (Amersham). Molecular detection of the (-251A/T) polymorphism in the IL-8 gene was performed by restriction fragment length polymorphism typing. This involved a combination of PCR amplification and digestion with restriction endonuclease Mun I followed by gel electrophoretic analysis. The PCR conditions consisted of an initial denaturation step at 94 oC, followed by 35 cycles of 94 oC for 50 sec, 61 oC 4 for 1 min, and 72 oC for 55 sec, as well as a final elongation step at 72 oC for 5 min. The primers used were Forward: 5’-ATCTTGTTCTAACACCTGCCACTCT-3’ and Reverse: 5’- TAAAATACTGAAGCTCCACAATTTGG-3’. The generated PCR product of 121 bp was cleaved by restriction enzyme Mun I into two fragments of 82bp and 39bp only if the A allele was present. The statistical analyses were performed using SAS® software (version 9.0; SAS Institute Inc.). The frequencies of alleles and genotypes (with the AT genotype as referent) of the whole group or subgroups of patients were compared to the respective frequencies of the control group using the Fisher’s exact test and odds ratios, while all genotype distributions were according to Hardy-Weinberg estimates. All statistical analyses concerning: number of relatives with a history of cancer, number of relatives with a history of thrombosis, nicotine use, alcohol use, have assumed that all controls have nil values for the above variables (i.e. all controls do not have a family history of cancer, all controls do not have a family history of thrombosis, all controls do not use tobacco, and all controls do not drink alcohol). Thus, odds ratios are most likely expected to overestimate the true likelihood of IL-8 genotypes and these variables. The Maentel - Haenzel method was used for the calculation of all odds ratios with a 95% confidence interval (CI). A Pvalue less than 0.05 was considered statistically significant. Results Demographic and lifestyle characteristics in healthy controls and patients with oral cancer are shown in Table 1. The data for the two tested populations (Greek and German healthy controls) were analyzed together, since there were no significant differences of allele frequencies of the -251A/T polymorphism among the two populations. The observed genotypes in the control group were AA=0, AT=84, TT=72, resulting in mutant allele A frequency of 23.1% (similar to other European populations) and carrier frequency of 46.2%. All -251A/T genotype distributions were as 5 expected in Hardy-Weinberg equilibrium in the control group, as well as in the whole group and subgroups of patients. The detected genotypes in the patients’ group were AA=14, AT=56, TT=88, resulting in significantly increased mutant allele A and carrier frequencies compared to the equivalent ones in the control group (36.7% (P<0.001) and 64.6% (P<0.05) respectively). Significant increase of both frequencies was also detected in certain subgroups of patients in comparison to controls (P<0.05). Specifically, statistical difference in mutant allele and carrier frequencies was observed in the subgroups of patients a) in early (I,II) stages of cancer (genotypes AA=14, AT=26, TT=46, P<0.001 and P<0.001, respectively b) without positive family history of cancer (genotypes AA=10, AT=32, TT=56, P<0.001 and P<0.001, respectively), c) without positive family history of thrombophilia (genotypes AA=12, AT=46, TT=68, P<0.001 and P<0.05, respectively), d) with tobacco abuse (genotypes AA=14, AT=52, TT=82, P<0.001 and P<0.05, respectively) and e) with alcohol abuse (genotypes AA=6, AT=10, TT=36, P<0.001 and P<0.001, respectively). Significant difference only in mutant allele A frequency was detected in the subgroups of patients with positive family history of cancer (genotypes AA=4, AT=24, TT=32, P<0.05), with positive family history of thrombophilia (genotypes AA=2, AT=10, TT=20, P<0.05) and without alcohol abuse (genotypes AA=8, AT=46, TT=52, P<0.05). Finally, there were no significant differences with controls in genotypes and allele frequencies of subgroups of patients with categorizations of advanced (III,IV) cancer stages (genotypes AA=0, AT=30, TT=42), non-smoking habits (genotypes AA=0, AT=4, TT=6), sex, age, and age at onset of oral cancer. Interestingly, compared to individuals with the TT genotype, the relative risk for oral squamous cell carcinoma for TA heterozygotes was 1.76 (1.11-2.79). Additionally, TA heterozygotes have a relative risk of 1.89 (1.05-3.40) for developing oral cancer in stages I&II and 1.6 (0.91-2.82) in stages III&IV. 6 Discussion The cytokine IL-8 has been correlated with some types of cancer due to its angiogenic properties and its ability to induce cell proliferation and promote DNA damage through inhibition of DNA repair18,19. Increased levels of IL-8 have been detected in oral squamous carcinoma cell lines and patients with ovarian and hepatocellular cancer15-17. Furthermore, elevated IL-8 expression has been associated with tumor growth and metastasis in melanoma and breast cancer20,21. The mutant allele A of the (-251A/T) polymorphism in the promoter region of the IL-8 gene increases production of IL-8 cytokine and has been associated with higher risk of prostate and gastric cancer13,14. In light of the above, the purpose of this study was to investigate whether the (-251A/T) polymorphism which affects IL-8 gene expression might be associated with risk for oral oncogenesis. The subjects under investigation were European patients with oral cancer whose genotypes were compared to those of healthy controls with adjustments made on age, sex and ethnicity. Despite the relatively small sample of studied individuals, the overall obtained data revealed a strong association of the high gene expression allele A with an increased risk for oral squamous cell carcinoma. By comparison to controls, significantly increased percentages of the mutant allele were detected in the studied group of patients, regardless of their family history of cancer or thrombophilia, as well as their stage of tumor. Higher levels of IL-8 may promote carcinogenesis not only by inducing cell proliferation and DNA damage, but by upregulating gene transcription and activity of metalloproteinase-2 (MMP-2, collagenase type IV) as well18-20. Increased collagenase activity results in enhanced invasion of surrounding healthy tissues by tumor cells, increased angiogenesis and therefore, metastasis20. Interestingly, the suppression of IL-8 expression by synthesized positive inotropic 7 agent vesnarinone inhibits both angiogenesis and tumorigenicity of human oral squamous cell carcinoma cells22. In contrast to the findings of the present report, a study of the same IL-8 polymorphism in a Chinese population did not detect any association with esophageal squamous cell carcinoma, indicating that diverse tumorigenic mechanisms possibly exist among the two tumors14. This notion is reinforced by findings of two studies in Chinese, in which a MMP-1 polymorphism was associated with oral cancer but not esophageal cancer23,24. The fact that some but not all the patients in this study had a mutant IL-8 allele may be explained by the possible contribution of similar polymorphisms affecting gene expression of other interleukins. For example, the -590 C/T polymorphism in the IL-4 gene is also associated with increased risk for oral cancer25. Furthermore, increased serum levels of interleukins IL-6 and IL-1β have also been observed in oral squamous cell carcinoma26. As a consequence, it is of great importance to perform further genetic association studies regarding the contribution of factors related to angiogenesis, inflammation and thrombosis in predisposition to oncogenesis in the oral region. Any positive findings could ultimately result in the undertaking of preventive measures safeguarding the health status and lives of certain at risk individuals in the general population. Acknowledgements This work was supported in part by EPEAEK “Pythagoras” grant 70/3/7391 of the Greek Secretariat of Research and Technology to E.V. 8 References 1. Williams HK. Molecular pathogenesis of oral carcinoma. J Clin Pathol 2000;53:165-72. 2. Song C, Xing D, Tan W, Wei Q, Lin D. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res 2001;61:3272-5. 3. Vairaktaris E, Yapijakis C, Wiltfang JV, , Ries J, Vylliotis A, Vasileiou S et al. Are factor V and prothrombin mutations associated with increased risk of oral cancer? Anticancer Res 2005 ;25:2561-6. 4. Vairaktaris E, Yapijakis C, Vylliotis A, Wiltfang J, Kessler P, Ries J et al. Methylenetetrahydrofolate reductase polymorphism and minor increase of risk for oral cancer. J Cancer Res Clin Oncol 2006;132:219-22. 5. Vairaktaris E, Yapijakis C, Serefoglou Z, Vylliotis A, Ries J, Nkenke E et al. Plasminogen activator inhibitor-1 polymorphism is associated with increased risk for oral cancer. Oral Oncol 2006. In press. 6. Vairaktaris E, Yapijakis C, Derka S, Serefoglou Z, Vylliotis, Jutta Ries J et al. Association of platelet Ia polymorphism with minor increase of risk for oral cancer. Eur J Surg Oncol 2006. In press. 7. Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD et al. Inhibition of Interleukin 8 Attenuates Angiogenesis in Bronchogenic Carcinoma. J Exp Med 1994;179:140915. 8. van Aken BE, Reitsma PH, Rosendaal FR. Interleukin 8 and venous thrombosis: evidence for a role of inflammation in thrombosis. Br J Haematol 2002;116:173-7. 9 9. Brennecke S, Deichmann M, Nacher H, Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res 2005;15:515-22. 10. Rollins BJ. Chemokines. Blood 1997;90:909-28. 11. Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner SG, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992;258:1798. 12. Hull J, Thomson and Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax 2006;55:1023-27. 13. McCarron SL, Edwards S, Evans PR, Gibbs R, Dearnaley DP, Dowe A et al. Influence of Cytokine Gene Polymorphisms on the Development of Prostate Cancer. Cancer Research 2002;62:3369-72. 14. Savage SA, Abnet CC, Mark SD, Qiao YL, Dong ZW, Dawsey SM et al. Variants of the IL8 and IL8RB Genes and Risk for Gastric Cardia Adenocarcinoma and Esophageal Squamous Cell Carcinoma. Cancer Epidemiol, Biomarkers Prev 2004;13:2251-7. 15. al-Wabel A, al-Knawy B, Raziuddin S. Interleukin-8 and granulocyte-macrophage colonystimulating factor secretion in hepatocellular carcinoma and viral chronic active hepatitis. Clin Immunol Immunopathol 1995;74:231-5. 16. Watanabe H, Iwase M, Ohashi M, Nagumo M. Role of interleukin-8 secreted from human oral squamous cell carcinoma cell lines. Oral Oncol 2002;38:670-9. 17. Lokshin AE, Winans M, Landsittel D, Marrangoni AM, Velikokhataya l, Modugno F et al. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecol Oncol 2006. In press. 10 18. Jaiswal M, LaRusso NF, Burgart LJ, and Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 2000;60:184-90. 19. Moore MA. Cytokine and chemokine networks influencing stem cell proliferation, differentiation and marrow homing. J Cell Biochem 2002;38:29-38. 20. Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M et al. Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis. Am J Pathol 1997;151:1105-13. 21. Bendre MS, Kurten DG, Foote TM, Akel NS, Skinner RA, Nicholas RW et al. Expression of Interleukin 8 and Parathyroid Hormone-related Protein by human Breast Cancer Cells Correlates with Bone Metastasis in Vivo. Cancer Research 2002;62:5571-79. 22. Harada K, Supriatno, Yoshida H, Sato M. Vesnarinone inhibits angiogenesis and tumorigenicity of human oral sqquamous cell carcinoma cells by suppressing the expression of vascular endothelial growth factor and interleukin-8. Int J Oncol 2005;27:1489-97. 23. Jin X, Kuang G, Wei LZ, Wang R, Guo W, Wang N et al. No association of the matrix metalloproteinase 1 promoter polymorphism with susceptibility to esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma in northern China. World J Gastroenterol 2005;11:2385-9. 24. Cao ZG, Li CZ. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances oral squamous cell carcinoma susceptibility in a Chinese population. Oral Oncol 2006;42:32-8. 25. Tsai MH, Chen WC, Tsai CH, Hang LW, Tsai FJ. Interleukin-4 gene, but not the interleukin-1 beta gene polymorphism, is associated with oral cancer. J Clin Lab Anal 2005;19:93-8. 11 26. Jablonska E, Piotrowski L, Grabowska Z. Serum Levels of IL-1b, IL-6, TNF-a, sTNF- RI and CRP in patients with oral cavity cancer. Pathol Oncol Res 1997;3:126-9. 12