Objectives 14
advertisement

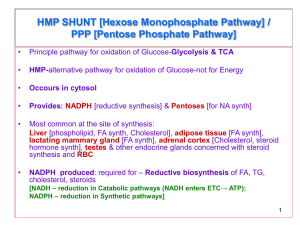
1. PENTOSE PHOSPHATE PATHWAY - source of NADPH for reductive synthesis of fatty acids and cholesterol in liver, kidney, and adipose - NADPH maintains glutathione in reduced state - can also provide source of ribose-5-phosphate nucleotide synthesis nucleic acids - provides for interconversion of pentoses with hexoses/trioses Oxidative branch (irreversible) - glucose-6-P 6-Phosphogluconate Ribulose-5-Phosphate (Ribose-5-phosphate) - key enzyme and rate-limiting step is glucose-6-P dehydrogenase; produces first of two NADPH, used NADP+ as cofactor; glucose-6-PDHase has regulation feed back inhibition by NADPH - second molecule of NADPH produces in oxidative branch is product of 6-phosphogluconate dehydrogenase catalyzes decarboxylation of hexose to a pentose sugar (ribulose-5-P) - end product is ribose-5-P precursor for synthesis of nucleic acids Non-oxidative branch (reversible) - includes sugar intermediates containing 4,5, or 7 carbons - transketolase catalyzes 2/3 of reaction in branch; requires thiamine diphosphate (TDP)as a cofactor (prosthetic group) as well as Mg2+ ions (as does PDH and alpha-ketoglutarate dehydrogenase) -thiamine (B1) deficiency affects both ribose-5-P production for nucleic acid synthesis and energy metabolism for carbohydrates; Wernicke-Korsakoff syndrome exhibits memory loss due decreased synthesis of nucleic acids in nervous tissue - high carb diet thiamine deficiency beriberi; tiring, weakness related to energy depletion; high carb diet individuals rely on pyruvate and alpha-ketoglutarate DHase for aerobic production of ATP from glucose - when non-oxidative branch begins with ribulose-5-P endproducts are glycolytic intermediates: glyceraldehyde-3-P and fructose-6-P; reversible reactions starting with these intermediates ending with ribose-5-P Cellular needs for ribose-5-P and NADPH - cell requires NADPH but not ribose-5-P - glucose-6-P from hexokinase in most tissues, but by glucokinase in liver/pancreatic beta-cells oxidative branch to ribulose-5-P converted to fructose-6-P and glyceraldehyde-3-P in 2:1 ratio intermediates can be metabolized to pyruvate in glycolysis or returned to glucose-6P via gluconeogenic pathway - shunting around part of glycolysis (hexose monophosphate shunt) - 2/3 of ribulose-5-P must be used to form xylulose-5-P with remaining 1/3 being converted to ribose-5-P - both oxidative and non-oxidative branches working Cell requires ribose-5-P for nucleic acid synthesis but does not require NADPH - fructose-6-P and glyceraldehyde-3-P provided from glycolysis in 2:1 ratio for non-oxidative branch - non-oxidative branch proceeds in opposite direction than figure 1 - ribose-5-P produced from glycolytic intermediates; oxidative pathway shutdown by NADPH feedback inhibition Cell requires both NADPH and ribose-5-P - cell operates only oxidative branch of pentose pathway - all ribulose-5-P converted to ribose-5-P 2. REACTIVE OXYGEN SPECIES (ROS) - toxic ROS generated during normal course of cellular metabolism; ROS inhibited through action of antioxidants - look at table for ROS and antioxidants that reduce them (14-4) Free radicals - unpaired electron; extremely reactive against compounds that contain double bonds like unsaturated lipid (plasma membrane) and nucleic acids bases - when damage occurs to membranes oxidized molecules recycled - antioxidants eliminate ROS before they cause damage Singlet oxygen – generated from molecular oxygen in presence of UV light and heme iron; found in skin; not a radical; reacts with biological molecules and generates oxygen radicals (superoxide); contributes to oxidative damage in cells Superoxide radical anion- superoxide; formed by transfer of a single electron to oxygen; accomplished via coenzyme Q in mitochondrial electron transport chain or by NADPH oxidase in WBCs Hydrogen peroxyl radical – produced by protonation of superoxide; participate in same reactions as hydroxyl radicals (less reactive) Hydrogen peroxide – central role in formation of ROS; produced from superoxide via superoxide dismutase (antioxidant) and Haber-Weiss reaction Hydroxyl radical – most reactive ROS; produced from superoxide via Haber-Weiss reaction or via spontaneous degradation of hydrogen peroxide catalyzed by free metal ions such as iron; basis for antibiotic actions of hydrogen peroxide Lipid peroxyl radical – depends on action of hydroxyl radicals; primary targets of hydroxyl radicals are membrane phospholipids because most fatty acids in these structures are unsaturated (double bonds) reaction forms a lipid radical intermediate rapidly oxidized by molecular oxygen to form lipid peroxyl radical further oxidizes membrane lipids regenerates more hydroxyl radicals 3. ANTIOXIDANT DEFENSE Glutathione and selenium Glutathione peroxidase: 2 GSH + H2O2 GSSG + 2 H2O - glutathione, a tripeptide (glu-cys-gly), exists predominantly in reduced form (GSH) when cys has free SH group - oxidized form (GSSG) consists of two tripeptides linked by a disulfide bond (-S-S-) - GSH oxidized to GSSG via glutathione peroxidase (uses hydrogen peroxide) - reduced glutathione (2 GSH) is a natural antioxidant - selenium required for activity; cysteine residue is active site modified to selenocysteine - GSH/GSSG = 100:1 Glutathione reductase: GSSG + NADPH + H+ 2 GSH + NADP+ - reductase contains bound FAD to transfer electrons from NADPH to GSSG - NADPH derived from pentose pathway (niacin is precursor of NADPH) Glutathione and pentose phosphate pathway - defect in glucose-6-PDHase in RBC defects in membrane stability - event that demands RBC for NADPH increase defect in G6DPH prevents cell from maintaining adequate amount of GSH to reduce peroxides - peroxides in blood can convert hemoglobin methemoglobin and destroy RBC membrane through oxidation of phospholipids RBC destruction rise in insoluble bilirubin in blood - bilirubin is a breakdown product of heme that is insoluble until processed by liver into a soluble form Catalase and superoxide dismutase - catalase: 2 H2O2 H2O + O2 - hydrogen peroxide catalytically removed by catalase - catalase contains four heme groups; enzyme found in liver, kidney, blood, and mucous membranes - often localized in peroxisomes of cell - hydrogen peroxide produced by catabolism of superoxide by superoxide dismutase that catalyzes reaction of 2O2-. + 2H+ H2O2 + O2 - superoxide dismutase in cytoplasm contains two identical subunits requiring Cu2+ and Zn2+ as cofactors; mitochondrial enzyme requires Mn2+ as a cofactor - although ROS hydrogen peroxide is form it is easily reduce to water by catalase - mutants in Cu,Zn-superoxide dismutase gene occurs in patients with familial amyotrophic lateral sclerosis (FALS), a fatal neurodegenerative disorder Antioxidant cascade Glutathione – reduced glutathione (GSH) protects cell against peroxides (glutathione peroxidase) and then is regenerated ultimately by NADPH produced in pentose phosphate pathway Vitamin C – - GSH plays an additional antioxidant role through its ability to regenerate reduced vitamin C (ascorbic acid) protects cells against oxidant effects of both hydroxyl radicals and superoxide radical anion by reducing them - reduced vitamin C restores vitamin E to its reduced state - vitamin C maintains iron in reduced state (in prolyl and lysyl hydroxylase) of collagen synthesis - deficiency scurvy - excess vitamin C intake production of oxalates formation of kidney stones as calcium oxalate Vitamin E - antioxidant role of reduced Vitamin E is to rid cell of lipid peroxyl radical; it also acts on singlet oxygen and peroxyl free radical - vitamin E is a fat-soluble vitamin; its major constituent is alpha-tocopherol - vitamin E deficiency (rare) leads to increased peroxidation of membrane lipids, decreased mitochondrial activity, DNA mutations, and damage to cell transport processes; fragility of RBCs