PICTURE - Michigan State University
advertisement

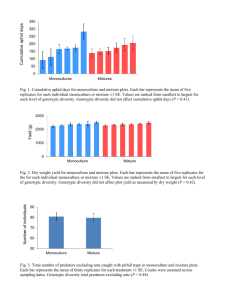
QuickTime™ and a decompressor are needed to see this picture. QuickTi me™ and a decompressor are needed to see thi s pi ctur e. Photographs of Toxomerus syrphid fly egg (Bugg 2008), larva (Bugg 2008) and adult (Anna Fiedler) Toxomerus spp. (Diptera: Syrphidae) By Brett Blaauw, Department of Entomology, Michigan State University, East Lansing, Mi 48823 Introduction Flies in the family Syrphidae are commonly known as syrphid flies, hover flies, or flower flies. Syrphidae is one of the most diverse families of Diptera with nearly 6000 described species, and members which can be found on every continent across the globe, except Antarctica. Within the family of Syphidae the subfamily Syrphinae consists of primarily aphidophagous (consumes aphids) species of syrphids. The genus Toxomerus, in the Syphinae subfamily, is a commonly observed syrphid genus with 16 known species found across North America. Numerous adult Toxomerus spp. are considered Batesian mimics of non-palatable models, such as stinging wasps and bees (Hymenoptera), and are frequently abundant on flowers, which are used as mating sites and food sources. Syrphid larvae prey on aphids, scale insects, and thrips, and are considered extremely beneficial since each larva is capable of consuming hundreds of aphids during its development. Appearance Syrphids in the genus Toxomerus have small, chalky white eggs that resemble a small grain of rice. Adult females lay eggs singly on leaves, usually in or near an aphid colony. The larvae of Toxomerus spp. can be found on aphid-infested leaves and are 4 to 5 mm long, translucent, green to cream colored, and have a visible digestive system. The larvae also have no eyes and are typically maggot-shaped; tapering to a point at the head end and broadly rounded at the rear. The mouthparts of Toxomerus spp. larvae consist of a triple-pointed stylet that is used to seize and pierce prey in order to suck them dry. Adult Toxomerus spp. are fast and agile flyers, often seen hovering over flowers. They are 5 to 9 mm in length and superficially resemble bees or wasps, but have a more flattened body and only one pair of clear wings. Although the tip of the abdomen is pointed in female Toxomerus syrphids (males have a more rounded), they do not have a stinger or chewing mouthparts, unlike bees and wasps , so they are harmless to humans. Syrphids can be distinguished from other flies by the presence of a spurious (i.e. “false”) vein on each wing that runs between the radial and medial veins (Figure 1). Other physical traits common to Toxomerus spp. adults are a brownish to yellowish scutellum (small triangular region between thorax and abdomen) and a thin abdomen with a continuous yellow margin or alternating black and yellow margin with dark horizontal bands extending all the way to the abdominal edges. They also have large, often redish eyes with a v-shaped notch on the back margin of each eye. Figure 1. Spurious vein of a syrphid fly wing. Habitat Larvae of Toxomerus syrphids do not have legs or eyes, and are therefore confined to plants where they were hatched, which are generally near or within colonies of their prey (e.g. aphid colony). Syrphid adults, on the other hand, are highly mobile flyer and can be found in a wide range of habitats. Adult Toxomerus spp. often inhabit open fields containing flowers or transition zones between woodlands and grasslands. They have also been known to commonly inhabit hedgerows and windbreaks within fields that contain flowering plants, which provide shelter as well as food. Pests Attacked Adult Toxomerus spp. are not predaceous and mainly feed on nectar and pollen. Syrphid fly larvae on the other hand, are voracious predators of aphids, scales, thrips, and even small caterpillars. It has also been observed that some species in the genus Toxomerus are even able to survive on plant tissue and sap, making them more generalists compared to most syrphid flies that primarily feed on aphids. Life Cycle Line drawing of Toxomerus spp. life cycle (Brett Blaauw). Toxomerus syrphids overwinter as late-instar larvae or pupae in the soil or in leaf litter. Adults begin emerging in April and May and require nectar and pollen from wild flowers in order to build up enough energy to begin mating and producing eggs (females). After mating, female syrphids oviposit eggs on leaves near or in host (e.g. aphids) colonies, where the young larvae will hatch and easily locate their prey. The larvae feed and grow for 7 to 10 days, and then drop to the soil or leaf litter to pupate. The syrphid life cycle is completed in 16 to 28 days and the adults can live up to a month, allowing for multiple generations each year. The final generation of larvae will overwinter as either late-instars or pupae, emerging the following spring to repeat the cycle. Relative Effectiveness Worldwide, predaceous syrphid larvae are an important naturally occurring control agent of aphids and other soft-bodied pest insects in agricultural systems, and in a few cases have even been documented as the principal aphid predator. The ability of Toxomerus spp. syrphid larvae to effectively suppress aphids depends on variety of factors, such as climate, time of emergence, habitat quality and the presence of flowers for pollen and nectar. Efforts to manipulate agricultural landscapes to provide shelter with hedgerows and food from flowering plants have been able to increase the supportive nature of agricultural systems for syrphids as well as other beneficial insects. Pesticide Susceptibility Toxomerus syrphid flies are highly susceptible to broad-spectrum insecticides, especially within the first 24 hours of insecticide application. It is recommend to avoid broad-spectrum insecticidal control of aphid infestations if you see syrphid flies within or adjacent to fields. Commercial Availability Smith and Chaney (2007) have described techniques for rearing Toxomerus spp. and other syrphids in the laboratory by collecting larvae and eggs from the field, and some species may be amenable to commercial production. However, the only currently available hoverfly for purchase in the United States is Episyrphus balteatus from Koppert Biological Systems. Acknowledgments Thanks to Anna Fiedler for suggesting this genus of syrphids and providing me with a great photograph of a syrphid adult. References Almohamad, R., F. J. Verheggen, F. Francis, and E. Haubrudge. 2007. Predatory hoverflies select their oviposition site according to aphid host plant and aphid species. Entomol. Exp. Appl. 125: 13-21. Arrigon, F., M. Deconchat, J. P. Sarthou, G. Balent, and C. Monteil. 2007. Modelling the overwintering strategy of a beneficial insect in a heterogeneous landscape using a multi-agent system. Ecol. Model. 205: 423-436. Bugg, R. L. 1991. Cover crops and control of arthropod pests of agriculture, pp. 157-163. In W. L. Hargrove [ed.], Cover crops for clean water. Soil and Water Conservation Society, Jackson, Tennessee. Bugg, R. L. 1992. Manipulation to enhance the effectiveness of aphidophagous hover flies (diptera: Syrphidae). (http://www.sarep.ucdavis.edu/NEWSLTR/v5n2/sa-11.htm). Bugg, R. L., and L. T. Wilson. 1989. Ammi visnaga (l.) lamarck (apiaceae): Associated beneficial insects and implications for biological control, with emphasis on the bell-pepper agroecosystem. Bio. Ag. and Hort.. 6: 241-268. Bugg, R. L., R. Colfer, W. E. Chaney, H. A. Smith, and J. Cannon. 2008. Flower flies (syrphidae) and other biological control agents for aphids in vegetable crops. Agirculture and Natural Resources Publication. 8285. Chaney, W. E. 2004. Insectary plants for vegetable crops, pp. 53-54. In M. Hodle [ed.], Proceedings of the California conference on biologicaal control iv. UCDANR, Davis, California. Coin, P. 2008. Genus Toxomerus. (http://bugguide.net/node/view/3277). Colfer, R. 2004. Using habitat management to improve biological control on commercial organic farms in california, pp. 55-62. In M. Hodle [ed.], Proceedings of thecalifornia conference on biologicaal control iv. UCDANR, Davis, California. Colley, M. R., and J. M. Luna. 2000. Relative attractiveness of potential beneficial insectary plants to aphidophagous hoverflies (diptera: Syrphidae). Environ. Entomol. 29: 1054-1059. Deans, A. M., S. M. Smith, J. R. Malcolm, W. J. Crins, and M. I. Bellocq. 2007. Hoverfly (syrphidae) communities respond to varying structural retention after harvesting in canadian peatland black spruce forests. Environ. Entomol. 36: 308-318. Ferrar, P. 1987. A guide to the breeding habits and immature stages of diptera cyclorrhapha. Scandinavian Science Press, Copenhagen. Flint, M. L., and S. H. Dreistadt. 1998. Natural enemies handbook: The illustrated guide to biological pest control. University of California Press, Berkley. Hendrickx, F., J. P. Maelfait, W. V. Wingerden, O. Schweiger, M. Speelmans, S. Aviron, I. Augenstein, R. Billeter, D. Bailey, R. Buckacek, F. Burel, T. Diekotter, J. Dirksen, F. Herzog, J. Liira, M. Roubalova, V. Vandomme, and R. Bugter. 2007. How landscape structure, land-use intensity and habitat diversity affect components of total arthropod diversity in agricultural landscapes. J. Appl. Ecol. 44: 340-351. Jansen, J. P. 2000. A three-year field study on the short-term effects of insecticides used to control cereal aphids on plant-dwelling aphid predators in winter wheat. Pest Manag. Sci. 56: 533-539. Kaiser, M. E., T. Noma, M. J. Brewer, K. S. Pike, J. R. Vockeroth, and S. D. Gaimari. 2007. Hymenopteran parasitoids and dipteran predators found using soybean aphid after its midwestern united states invasion. Ann. Ent. Soc. America. 100: 196-205. Koppert, B. V. 2009. Syrphidend - koppert biological control natural pollination. (http://www.koppert.com/Aphid.13955.0.html). Luna, J. M., and P. Jepson. 2001. Enhancement of biological control with insectary plantings. , Organic Farming Research Foundation Project Report. Dept. of Horticulture, Oregon State University, Corvallis, Oregon. Markova, E., and E. Ljubenova. 1998. Influence of the synthetic pyrethroid insecticide alphacypermethrin on the structure of the syrphid coenosis (syrphidae, diptera) in a potato ecosystem. J. Appl. Entomol. 122: 469-473. Schmidt, M. H., U. Thewes, C. Thies, and T. Tschamtke. 2004. Aphid suppression by natural enemies in mulched cereals. Entomol. Exp. Appl. 113: 87-93. Schmidt, N. P., M. E. O'Neal, and P. M. Dixon. 2008. Aphidophagous predators in Iowa soybean: A community comparison across multiple years and sampling methods. Ann. Ent. Soc. America. 101: 341-350. Schneider, E. 1969. Bionomics and physiology of aphidophagous syrphidae. Annu. Rev. Entomol. 14: 103-123. Smith, H. A., and W. E. Chaney. 2007. A survey of syrphid predators of Nasonovia ribisnigri in organic lettuce on the central coast of california. J. Econ. Entomol. 100: 39-48. Smith, H. A., W. E. Chaney, and T. A. Bensen. 2008. Role of syrphid larvae and other predators in suppressing aphid infestations in organic lettuce on california's central coast. J. Econ. Entomol. 101: 1526-1532. Steinkraus, D. C., and J. P. Kramer. 1987. Susceptibility of sixteen species of diptera to the fungal Pathogenentomophthora muscae (Zygomycetes: Entomophthoraceae). Mycopathologia. 100: 55-63. Tooker, J. F., M. Hauser, and L. M. Hanks. 2006. Floral host plants of syrphidae and tachinidae (diptera) of central illinois. Ann. Ent. Soc. America. 99: 96-112. Van Emden, H. F. 1966. The effectiveness of aphidophagous insects in reducing aphid populations. In I. Hodek [ed.], Ecology of aphidophagous insects. Academia, Prague. Weems, H. V., Jr. 1954. Natural enemies and insecticides that are detrimental to beneficial syrphidae. The Ohio Journal of Science. 54: 45-54.