Chemistry is the study of chemicals and their properties, and the
advertisement
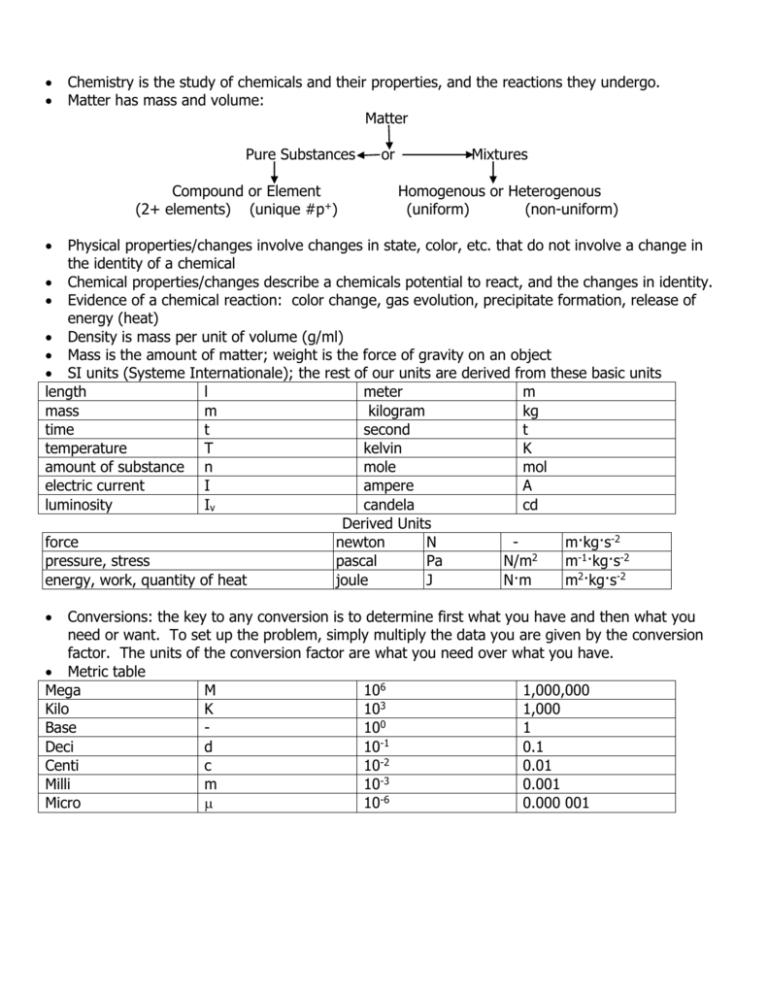
Chemistry is the study of chemicals and their properties, and the reactions they undergo. Matter has mass and volume: Matter Pure Substances Compound or Element (2+ elements) (unique #p+) or Mixtures Homogenous or Heterogenous (uniform) (non-uniform) Physical properties/changes involve changes in state, color, etc. that do not involve a change in the identity of a chemical Chemical properties/changes describe a chemicals potential to react, and the changes in identity. Evidence of a chemical reaction: color change, gas evolution, precipitate formation, release of energy (heat) Density is mass per unit of volume (g/ml) Mass is the amount of matter; weight is the force of gravity on an object SI units (Systeme Internationale); the rest of our units are derived from these basic units length l meter m mass m kilogram kg time t second t temperature T kelvin K amount of substance n mole mol electric current I ampere A luminosity Iv candela cd Derived Units force newton N m·kg·s-2 2 pressure, stress pascal Pa N/m m-1·kg·s-2 energy, work, quantity of heat joule J N·m m2·kg·s-2 Conversions: the key to any conversion is to determine first what you have and then what you need or want. To set up the problem, simply multiply the data you are given by the conversion factor. The units of the conversion factor are what you need over what you have. Metric table Mega M 106 1,000,000 3 Kilo K 10 1,000 0 Base 10 1 Deci d 10-1 0.1 -2 Centi c 10 0.01 -3 Milli m 10 0.001 -6 Micro 10 0.000 001 Energy is the capacity to do work and comes in 6 forms: chemical, electrical, light, mechanical, sound, heat Heat is the exchange of energy from high to low; temperature is the measure of how hot or cold something is Law of Conservation of Energy: energy is conserved Heat of Fusion and Heat of Vaporization are at the melting and boiling points of a compound respectively Specific Heat is the amount of heat needed to raise one gram of a compound by 1 degree Celsius or Kelvin (cp=q/m.T) Significant figure rules: o Non-zero digits are always significant. o Any zeros between two significant digits are significant. o A final zero or trailing zeros in the decimal portion ONLY are significant. 0.0001 has only 1 significant figure 0.00010 has 2 significant figures 1.0001 has 5 significant figures 200 has only 1 significant figure 200. has 3 significant figures o When quantities are added or subtracted, the number of decimal places in the answer is equal to the number of decimal places in the quantity with the smallest number of decimal places o In multiplication and division, the result may have no more significant figures than the factor with the fewest number of significant figures. Accuracy is how close data is to the true value; Precision is how close the data in repetitive trials are Endothermic reactions absorb heat to proceed; Exothermic reactions release heat o Reactant + heat Product decrease in temperature of surroundings o Reactant Product + heat increase in temperature of surroundings System: the entire group or sample set; Surroundings: the environment outside of the system Law of Conservation of Mass: Mass is conserved Democritus: Atoms are the smallest fraction of a molecule Three Laws of Atoms o Law of Definite Proportions o Law of Multiple Proportions o Law of Conservation of Mass Dalton’s Atomic Theory: o All matter is composed of atoms that cannot be subdivided, created or destroyed o Atoms of an element have the same properties o Atoms of different elements have different properties o Compounds are formed when different atoms combine in simple, whole numbers o In a chemical reaction, atoms combine, separate or rearrange, but are not created, destroyed or changed Atoms are made up of subatomic particles called protons, neutrons and electrons J.J. Thomson set up a crookes tube with a anodic and cathodic ends o When electricity was applied to the tube, a beam was emitted from the cathodic (-) plate o He tested the tube further by applying an electrical field to the tube using paddles o He concluded that the particles in the tube were negatively charged and had mass electrons, e, e-, -1.602 x 10-19C; mass = 9.109 x 10-31kg o J.J. Thomson’s Plum Pudding Model Robert Milikan calculated the charge of an electron Ernest Rutherford conducted experiments to test the Thomson model o He directed alpha particles through a thin gold foil and measured them with a film o There must be a dense region with positive charges (protons) surrounded by the electrons; nucleus o Rutherford concluded that the nucleus had all of the positive charge and most of the mass, but only a fraction of the volume o Most of the volume of an atom is empty space The Atomic Number is the number of protons in the nucleus and is unique for each element o The Atomic Mass Number is the number of Protons + Neutrons o Atomic Mass Number = Atomic Number + Number of Neutrons Different elements can have the same mass number, but different atomic number You cannot use mass number to identify an element The structure of the atom can be written using the element symbol, atomic number and mass number An Isotope has the same atomic number but different mass number (different number of neutrons) o Isotopes can be identified with the symbol, mass and atomic number o 3/2He or Helium-3 The Rutherford model described electrons as a cloud of negatively charged particles surrounding the nucleus If electrons are negatively charged, and the nucleus is positively charged, why don’t the electrons collide with the nucleus? Opposite charges attract? Niels Bohr was a quantam physicist who described energy levels of an electron o The Bohr Model is probably familar as the "planetary model" of the atom, the figure is used as a symbol for atomic energy o In the Bohr Model the neutrons and protons occupy a dense central region called the nucleus, and the electrons orbit the nucleus much like planets orbiting the Sun Louis deBroglie – responsible for connecting the wave theory of particles to the modern atomic theory Schrodinger Wave Theory: wave-particle duality; electrons exhibit both particle and wave like properties Plank stated that energy is absorbed and released in discrete units called quanta An orbital is a region of space that an electron is most likely to occur o S-orbitals: are spherical in shape and there is only 1; there are on each principal energy level o P-orbitals: there are 3 types of p-orbitals starting on the 2nd level o D-orbitals: there are 5 types starting on the 3rd level o F-orbitals: there are 7 types starting on the 4th level o Think of orbitals as different types of rooms, each room can only hold 2 electrons Floor # Number of Maximum Number of (principal energy Type of Orbitals Orbitals Electrons level) 1 S 1 2 2 S, P 4 8 3 S, P, D 9 18 4 S, P, D, F 16 32 2 2 n n types n orbitals 2n electrons Electron Hotel 4th Floor 3rd Floor 2nd Floor 1st Floor F orbital types D orbital types P orbital types S orbital types 14 electrons + 10 electrons + 6 electrons + 2 electrons = 32 electrons total D orbital types P orbital types S orbital types 10 electrons + 6 electrons + 2 electrons = 18 electrons total P orbital types S orbital types 6 electrons + 2 electrons = 8 electrons total 2 electrons S oribital types Quantum numbers are unique numbers assigned to each individual electron which indicate the location and the energy of an electron The principal quantum number (n = 1, 2, 3, 4 ...) is the principal energy level Hund’s Rule: electrons entering an orbital-type (sublevel) will half-fill the orbitals in the sublevel before they fill it completely Pauli’s Exclusion Principle: no two electrons can exist in the exact same state; with the same quantum number Aufbau’ Principle: electrons occupy the lowest possible energy level An electron configuration is the arrangement of electrons in an atom The diagonal rule: fill each orbital type according to the direction of the arrows 6s 6p 6d 5s 5p 5d 5f 4s 4p 4d 4f 3s 3p 3d 2s 2p 1s Henry Mosely – periodic law; elements are arranged in the periodic table by their atomic number = # of protons = # of electrons The most modern form of the periodic table was introduced by Mendeleev. Main group elements: Group 1: Alkali Earth Metals Group 2: Alkaline Earth Metals Group 17: Halogens Group 18: Noble Gases Groups 3-12: Transition Metals Lanthanides and Actinides are the “f” orbital type elements Metals are on the left; non-metals are on the right; metalloids are along the stairstep Trends Ionization Energy, Ionic Radius and Electronegativity Elements are arranged in columns (group or families) and rows (periods) Families have similar characteristics; they are said to have periodicity New elements can be predicted based on these trends Atomic Radius Ionic Compounds are formed from a metal and non-metal; the difference in electronegativity is greater than 2.1 Most ionic compounds are solid at room temperature because of the strength of the bond An ionic compound contains repeating units or formula units bound together in a crystal lattice Lattice energy is the amount of energy needed to form or break an ionic bond Ionic compounds dissociate in water to form ions; these ions conduct electricity Electrons are exchanged in order to form a compound Ionic compounds are named with the cation (“+”) and then the anion (“-“) The anion is the name of the element + “-ide” If the anion is a polyatomic ion, simply name the ion Ions and parent atoms have different properties Octet rule; rule of 8: all compounds want to be more like a noble gas, they want an electron configuration like a noble gas (8 valence electrons). Therefore, they will lose/gain electrons to be stable Covalent Compounds are formed from a metal to metal or a non-metal and non-metal; the difference in electronegativity is less than 2.1 Polar covalent bonds are between 0.5 and 2.1 Non-polar covalent bonds are less than 0.5 Electrons are shared in order to form a compound As bond length increases, bond energy decreases A triple bond is the shortest and strongest of all the bonds Bond energy is the amount of energy needed for form or break a covalent bond Covalent compounds are named with the less electronegative atom first followed by the more electronegative atom. Prefixes are used to indicate ratios of atoms Lewis dot structures illustrate the configuration of the valence (outer shell) electrons The shape of a bond can predict the overall polarity of a compound (VSEPR or Valence Shell Electron Pair Repulsion) If there is a dipole, or an uneven shift of charge (remember that a compound cannot have an overall charge, so there is an unequal sharing of the + and – charges), then the compound is polar Polar dissolved polar and vice-versa VSEPR Chart VSEPR AB or AB2 AB2E AB3 AB4 AB3E AB2E2 AB5 AB6 SHAPE Linear Bent Trigonal-Planar Tetrahedral Trigonal-Pyramidal Bent Trigonal-Bipyramidal Octahedral The Mole is a counting unit; 6.022 x 1023 molecules, particles or atoms The atomic mass number, atomic weight/mass or molecular mass is the mass of 1 mole of atoms (by the way, you do know where to find the molecular mass? Try the periodic chart!) In Stoichiometry, the key to solving the quandry is the mole A balanced chemical equation indicates in simple, small, whole numbers the ratio of compounds to each other If you are given the mass or the volume of only one of the compounds in a chemical reaction, the remaining information can be calculated or derived using the principles of stoichiometry Here’s the deal: in a perfect world and on a perfect planet, compounds will come together an react in simple whole numbers. We, however, do not exist in said perfect world. Therefore, we must rely on the simple, small, whole number ratio of compounds Example: 6CO2 + 6H2O + energy C6H12O6 + 6O2 (by the way, this is photosynthesis; look familiar in reverse?) Okay, let’s read this reaction. Six moles of carbon dioxide and 6 moles of water and energy (in the form of light) react to form 1 mole of glucose and 6 moles of oxygen The ratio of these compounds is 6:6:1:6 So, if I start out with 51.4 grams of CO2 divided by the molecular mass which is 44 g/mol I get a total of 1.17 moles of CO2 to start with. So then, the ratio looks something like this: 1.17 : 1.17 : 0.195 : 1.17 How, you might ask? Well, it’s called a ratio, and we divide. 6:1 is the same thing as saying 1 : 1/6 Anyway, that’s how you figure out exactly how many moles of each compound you have. Then from there, you can multiply by the molecular mass to get actual mass Yield is the amount of product that you would expect to recover from a specified amount of reactant. Theoretical because we perform the experiment on paper first. The limiting factor is the reactant that theoretically produces the least amount of product. Reaction types: Synthesis: make a new compound from elements or simple substances Decomposition: break a complex compound into smaller or elemental parts Combustion: a hydrocarbon with oxygen as a fuel burn or combust to form CO2 and H2O Single replacement: only one element or polyatomic ion is replaced, exchanged or displaced. The order of this reaction depends upon the activity chart Double replacement: two elements or polyatomic species are replaced; the product must include a solid; this can be determined by the solubility guidelines Molar Heat Capacity (C) is the heat required to raise 1 mol of a substance by 1 degree K or C. C = q / n . T ; and o Specific heat (cp) is the heat required to raise 1 gram of a substance by 1 degree K or C. cp = q / m . T; so, o C = cp . M (where M is the molar mass in g / mol) Thermodynamics is the study of energy and energy is the capacity to do work. The 1st Law of Thermodynamics is also the Law of Conservation of Energy: Energy cannot be created or destroyed, it is conserved in a chemical reaction. Enthalpy (H) is the amount of heat absorbed or released in a chemical reaction and is measured in kJ / mol. o Enthalpy of formation Hof is the enthalpy to form a compound from its elements at standard thermodynamic conditions; 25oC and 1 atm. o Therefore, an element has an enthalpy of formation of “0”. Calorimetry is the experimental measure of an enthalpy change and is measured in kJ (calorie or kilocalorie is another unit) In an Exothermic Reaction, the change in enthalpy from the reactants to the product, results in a negative change in enthalpy. The potential energy for the products is less than the potential energy for the reactants. o In this reaction, the energy is on the product side (A + B C + 22 kJ) Potential Energy (kJ/mol) Exothermic Reaction 90 80 70 60 50 40 30 20 10 0 H = Hproducts - Hreactants Activated Complex H = C . T Activation Energy H =-22 kJ Energy of Reactants 1 2 3 4 5 6 Energy of Products 7 8 9 10 Reaction Coordinate (time) Hess’s Law states that the amount of heat released or absorbed does not depend on the number of steps; the sum of all changes in enthalpy equals to the net change in enthalpy. In an Endothermic Reaction, the change in enthalpy from the reactants to the product, results in a positive change in enthalpy. The potential energy for the products is greater than the potential energy for the reactants. o In this reaction, the energy is on the reactant side (A + B + 30 kJ C) Potential Energy (kJ) Endothermic Reaction Activation Energy 90 80 70 60 50 40 30 20 10 0 H=30 kJ Energy of Products Energy of Reactants 1 2 3 4 5 6 7 8 9 10 Reaction Coordinate (time) Entropy (S) is the measure of disorder in a system and is measured in J / K. The following events lead to an increase in disorder (entropy): o Increase in temperature; o Phase change from solid to liquid to gas; o More products than reactants; o Simpler products than reactants; o Substances that are put into solution; o Solutions that become more dilute (molecules have more space); o Gases that the pressure decreases (increases the volume and space). S = Sproducts - Sproducts Molar entropy (So) is the entropy of one mole of a compound. Gibb’s Energy (G), also called Gibb’s Free Energy, is the energy in a system available for work and is measured in kJ / mol. The amount of Gibb’s energy predicts a spontaneous reaction which is a reaction that occurs without any additional energy. A negative G is spontaneous and a positive G is not spontaneous; o If, H = + + and S = + + - then G= and the reaction is spontaneous ? spontaneous, low T ? spontaneous, high T + not spontaneous The factors that affect Gibb’s energy are enthalpy, entropy and temperature o G = H - TS; and o G = Gproducts - Greactants The 2nd Law of Thermodynamics states that in a spontaneous reaction, entropy is not conserved and must always increase. All spontaneous reactions contribute a portion of energy to perform useful work (Gibb’s Energy). o As a reaction proceeds, G decreases until it equals “0” which is the point of equilibrium. The states of matter are solid, liquid, gas and sometimes plasma (which is not a naturally occurring state) Cohesion is an attraction for particles that a liquid has. Adhesion is an attractive force for particles of solid surfaces Capillary action is the motion of a liquid up a small surface and is accomplished by adhesion of liquid molecules to the surface of the glass as well as cohesion between the liquid molecules. Surface tension is the fore that acts of the surface of a liquid and tends to minimize the area of the surface. Why? 1st of all, cohesive forces bring the molecules of a liquid together so that they stay in contact; 2nd, under the surface of the liquid, these cohesive forces are pulling equally in all directions; 3rd, only on the surface, the molecules are being pulled sideways and downward creating surface tension. It takes energy to increase the surface area of a liquid because this energy must oppose the net forces pulling the molecules; conversely, a liquid decrease energy as the surface area decreases. This tendency toward decreasing the surface area is called surface tension. A high surface tension means that a lot of energy is needed to break the surface. Application: surface tension is used in laundry. When you put dirty clothes in the washing machine and no laundry detergent, the dirt on your clothes cannot penetrate the surface tension of the water and so it stays on your clothes. When you add the detergent, the soap decreases the surface tension by disrupting hydrogen bonds and therefore the dirt can be carried away by the water!!! Although, it also takes effort to load the washing machine! Endothermic Reactions Gases do not have the same type of intermolecular forces, because they are farther apart and the attractive forces are minimized. That is why a gas will fill the space available. Now let’s talk about the physical changes in the states of matter. Refer to the diagram below. The direction of the arrow indicates the change from one state to another by the addition of release of energy. As energy is absorbed, the state changes from solid to liquid; liquid to gas; or solid to gas. As energy is released the state changes in the opposite direction. The name of each process is labeled. Evaporation Sublimation Melting Gas Condensation Liquid Deposition Freezing Solid Exothermic Reactions The process by which a solid changes into a liquid by the absorption of energy or the decrease in pressure is melting and the temperature that this occurs is the melting point. At this temperature, matter has absorbed enough energy for the molecules to break free from the attractive forces of the solid. The process by which a liquid changes into a solid by the release of energy or the increase in pressure is freezing and the temperature is called the freezing point. At this temperature, the particles of a molecule are slowed to a point where the intermolecular forces of attraction hold them in a crystalline state. Covalent bond types are a result of intermolecular forces (or van der Waals forces) or the forces of attraction that hold molecules together. This is a review. See the chart below for a summary: Bond Type Polar Covalent Bonds Hydrogen Bonds Non-Polar Covalent Bonds Ionic Bonds Intermolecular Forces (covalent) Dipole – Dipole Forces Explanation of Forces Positive and negative ends attract each other. The greater the electronegativity difference, the more polar the bond is. The more polar a bond, the stronger the attractive forces, stronger the bond. Stronger dipole forces Hydrogen bonds with very electronegative between neighboring atoms as Oxygen, Nitrogen or Fluorine of molecules another molecule and leaves the Hydrogen with a large partial positive charge. Because hydrogen only has one electron, which leaves behind a very strong proton London Dispersion Forces Weak attraction caused by temporary dipoles from the uneven distribution of electrons; interactions occur between the negative part of one molecule and the positive region of a neighboring molecule. Electrostatic Forces Cation and anion attractions • • • • • • • • • • • • The stronger the intermolecular forces and the stronger the bond, the greater the energy needed to break the bond. Molecules with stronger bonds have higher boiling and melting points and tend to be solids or liquids. Water has the ability to form two hydrogen bonds per one molecule of water which leads to greater versatility. London dispersion forces increase with increasing molecular mass and decrease with increasing distance between nuclei; in other words, the melting and boiling points increase with increase in mass. ORDER OF STRENGTH OF INTERMOLECULAR FORCES hydrogen bonding – strongest dipole – ion or electrostatic forces in ionic bonding dipole – dipole London dispersion forces Nonpolar substances are usually gases at room temperature or have low boiling points because of the low intermolecular forces Polar substances have high boiling points – many are solids at room temp. (ionic compound – strong intermolecular forces) Properties affected by intermolecular forces Boiling point Retention of volume and shape Surface tension Evaporation Vapor pressure Viscosity The enthalpy of fusion (symbol: ΔfusH), also known as the heat of fusion, is the amount of thermal energy which must be absorbed or evolved for 1 mole of a substance to change states from a solid to a liquid or vice versa. It is also called the latent heat of fusion or the enthalpy change of fusion, and the temperature at which it occurs is called the melting point. Hfus = H(liquid at melting point) – H(solid at melting point) When you withdraw thermal energy from a liquid or solid, the temperature falls. When you add heat energy the temperature rises. However, at the transition point between solid and liquid (the melting point), extra energy is required (the heat of fusion). To go from liquid to solid, the molecules of a substance must become more ordered. For them to maintain the order of a solid, extra heat must be withdrawn. In the other direction, to create the disorder from the solid crystal to liquid, extra heat must be added. The standard enthalpy change of vaporization, ΔvHo, also (less correctly) known as the heat of vaporization is the energy required to transform a given quantity of a substance into a gas. It is measured at the boiling point of the substance, although tabulated values are usually corrected to 298 K: the correction is small, and is often smaller than the uncertainty in the measured value. Values are usually quoted in kJ/mol, although kJ/kg, kcal/mol, cal/g and Btu/lb (obsolete) are also possible, among others. Hvap = H(vapor at boiling point) – H(liquid at boiling point) Molar entropy of vaporization, Svap are for the gaseous states. The standard enthalpy change of condensation (or heat of condensation) is numerically exactly equal to the standard enthalpy change of vaporization, but has the opposite sign. Enthalpy changes of vaporization are always positive (heat is absorbed by the substance), whereas enthalpy changes of condensation are always negative (heat is released by the substance). Melting point = Tmp = Hfus / Sfus and; Boiling point = Tbp = Hvap / Svap A phase is a uniform collection of particles. Some things can exist in two phases (milkshakes, slushies, etc.) Equilibrium is where G = 0; forward and reverse reactions occur at the same rate and the concentration of each stays the same. Dynamic equilibrium is a state of a compound where the particles move between 2 different states. Vapor pressure is the pressure produced by a liquid or a solid when it is in dynamic equilibrium with its gas phase and is measured in mmHg or kPa. As temperature increases, vapor pressure increases and exerts pressure on the walls of the container. PA phase diagram shows the state of a compound with temperature and pressure. The triple point is the temperature and pressure where the three phases exist in equilibrium The critical point (critical temperature and critical pressure) is the point above which the liquid and gas phases are indistinguishable (a supercritical fluid). Above the critical temperature, a gas cannot be liquefied. As vapor pressure increases, density increases. Pressure plays a greater role in the phases of liquids and gases than solids. To draw a phase diagram, plot the critical point, the triple point and the mp/bp. Solution: a homogenous mixture of two or more substances in a single phase; a solute and a solvent. Solute: a substance which is the portion of a solution that is dissolved. Solvent: a substance which is the portion of a solution that dissolves the solute; determines the phase of the solution. How does a solvent dissolve the solute, you might ask? The solvent has enough energy to break the surface tension or the bonds of the solute. Remember those pesky intermolecular forces?! The strongest of all are the metallic bonds followed by the ion-dipole, hydrogen, dipole-dipole, and finally the London dispersion forces. Table 2: Phases of solutions. Phase of the Phase of the Solute Solvent Solid Solid Solid Liquid Phase of the Solution Solid Liquid Example Metal alloy Kool-aid Liquid Gas Gas Liquid Liquid Gas Liquid Liquid Gas Ethanol-water Dr. Pepper Air Suspension: A mixture whose particles are evenly dispersed in a gas or a liquid and which settle Colloid: a mixture whose particles are smaller than a suspension but larger than a solution and does not settle; the particles are suspended in a liquid, gas or solid. The Tyndall effect is a out over time. Examples: sand – water mixture, cement phenomenon where a beam of light is reflected off of the particles and visible to the naked eye. Examples: milk, mayonnaise, marshmallow, fog One difference in mixtures and compounds is that mixtures can be separated into it’s parts. Mixtures can be separated by chromatography, gel electrophoresis, distillation, mechanical separation, evaporation, etc. Can you think of any more? Concentration is the measure of the amount of a particular substance in a given volume of solution. Table 3: Concentration calculations Name Units Application Molarity M mol solute / L solution Solution concentration Molality m mol solute / kg solvent Calculations using solids Normality N Molarity x n Titration calculations Parts per Million ppm g solute / 1 000 000 g solution To express very small 6 mass solute / mass solution x 10 concentrations Percentage by Weight % Mass solute/ mass solution x 100 % by weight Percentage by Vol. % Vol. solute / vol. solution x 100 % by volume Molarity: The molar unit is probably the most commonly used chemical unit of measurement. Molality: The molal unit is not used nearly as frequently as the molar unit. A molality is the Molarity is the number of moles of a solute dissolved in a liter of solution. A molar solution of sodium chloride is made by placing 1 mole of a solute into a 1-liter volumetric flask. Water is then added to the volumetric flask up to the one liter line. The result is a one molar solution of sodium chloride. M = n/V (remember that n = mass / molar mass) M = 1 mol NaCl / 1 L H2O & 1 mol NaCl = x grams NaCl / 58.44 g/mol NaCl So, M = 58.44 grams NaCl / 1 L H2O number of moles of solute dissolved in one kilogram of solvent. Be careful not to confuse molality and molarity. Molality is represented by a small "m," whereas molarity is represented by an upper case "M." Note that the solvent must be weighed unless it is water. One liter of water has a specific gravity of 1.0 and weighs one kilogram; so one can measure out one liter of water and add the solute to it. Most other solvents have a specific gravity greater than or less than one. Therefore, one liter of anything other than water is not likely to occupy a liter of space. To make a one molal aqueous (water) solution of sodium chloride (NaCl) , measure out one kilogram of water and add one mole of the solute, NaCl to it. The formula weight for NaCl is 58, and so 58.44 grams of NaCl dissolved in 1kg water would result in a 1 molal solution of NaCl. m = 1 mol NaCl / 1 kg H2O = 58.44 g NaCl / 1 kg H2O Percent by weight: To make up a solution based on percentage by weight, one would simply determine what percentage was desired (for example, a 20% by weight aqueous solution of sodium chloride) and the total quantity to be prepared. If the total quantity needed is 1 kg, then it would simply be a matter of calculating 20% of 1 kg which, of course is: 0.20 NaCl * 1000 g/kg = 200 g NaCl/kg. In order to bring the total quantity to 1 kg, it would be necessary to add 800g water. Making dilutions is easy. The starting solution is referred to as the stock solution and the solution that you make is referred to as the working solution. It is easier to make and store smaller volumes of concentrated stock solution. When you need a reagent (A substance used in a chemical reaction to detect, measure, examine, or produce other substances;) simply prepare it from a stock solution. The molarity and volume of the stock solution are inversely proportional. As you reduce one, you increase the other by the same proportion. Therefore; MstockVstock = MworkingVworking For example: You start out with a 12M concentrated solution of saline solution. The experiment calls for 0.8 Liters of a 3.0M solution. How would you prepare it? 12M x Vol. Stock = 3.0M x 0.8L Vol. Stock = 3.0M x 0.8L / 12M = 0.2L Therefore, you take 0.2L of your stock solution and dilute to 0.8L final volume. What makes a substance soluble? Solubility is the relative ability of a substance to dissolve into another at a given temperature and pressure; or the amount of solute that will dissolve into a given solution to saturate the solution. A saturated solution cannot dissolve any more solute – like a glass of iced tea that you added too much sugar to and the sugar settles on the bottom. An unsaturated solution contains less solute that the saturated solution – like the perfect glass of sweetened iced tea, with no settled solute (sugar) on the bottom. A supersaturated solution holds more dissolved solute than what is required to reach solubility equilibrium at a given temperature - or a solution that contains more solute than it would if the dissolved solute were in equilibrium with the undissolved solute. A solubility equilibrium is the state at which a solution is in equilibrium with its solute ions and the solvent. SO, try this at home. Saturate a pan of water with sugar, add more sugar so that it settles on the bottle. Heat the water. What happens to the excess sugar? (It dissolves into the water.) Allow the water to cool. Did the excess sugar fall out of solution? (No) Now, that is a supersaturated solution! The speed of solubility depends on the intermolecular forces, mass, surface area, temperature. As temperature increases, solubility increases. What effects do a solute have on a solvent? To answer that question, consider what happens when you apply salt (NaCl) to an icy road. Does the salt melt the ice? No, what the salt does is lower the melting or freezing point of water . What happens when you add salt to a pot of water on the stove? It raises the boiling point of the water. So using this example, a solute (the salt) decreases the melting/freezing point of the solvent (water) and raises the boiling point. The melting/freezing and boiling points of a solution are colligative properties. A colligative property is a property of a solution that is dependent upon the number of solute particles present and the nature of the solvent. These properties are independent of the identity of the solute. In other words, if you replaced 1 mole salt with 1 mole of sugar in the previous example, the effects on mp/fp and bp would be the same! The greater the concentration of solute, the greater the effect it has on a colligative property. A solute also lowers the vapor pressure of a given solvent. The change in the bp and mp can be calculated for solutions if the solute is molecular (covalent bonding, not ionic) and non-volatile (does not evaporate) because the bp and fp are proportional to the molality. Tb = (+kb) x (m) and; Tf = (-kf) x (m) where “+” indicates and increase and “-“ indicates a decrease in temperature. The symbols kb and kf are the boiling point and freezing point constants (oC/mol) and represents the number of degrees in Centigrade that the bp or fp is raised or lowered when 1 mole of a molecular, non-volatile solute is dissolved in 1 kg of a solvent. Henry’s Law states that at a constant temperature, the solubility of a gas is directly proportional Sometimes, solutions exhibit different characteristics than the parent solute or solvent. (Does that sound familiar? How about mother and daughter ions!) An electrolyte is a solute that is able to conduct electricity when dissolved in a solvent. It is said to have conductivity. Conversely, a non-electrolyte does not conduct electricity in solution or out of solution. Ions can be produced in solution 2 ways: 1. An ionic compound is separated into it’s ions by a process called dissociation; or 2. A polar covalent compound dissolves in water and loses a Hydrogen to form a Hydronium ion: HCl(g) + H2O(l) H3O+(aq) + Cl-(aq) The effect of these electrolytes on the bp and fp are higher or lower than expected based on the k x m equation. Why? Because, 1 mole of an ionic compound produces more than 1 mole of electrolytes! Let’s see this in action. One mole of NaCl produces 1 mole of Na+ and 1 mole of Clfor a total of 2 moles of electrolytes. Therefore, the effect on the bp/fp is 2 times the effects of a molecular substance. to the partial pressure of the gas on the surface of the liquid. When you open a soda, either in a bottle or in a can, there is a sudden release of pressure and the gas dissolved on the surface of the soda, comes out of solution (it foams up.) Soda is a saturated solution of CO 2 and water, sugar, etc. The carbon dioxide is exerting partial pressure on the surface of the soda. Soda has a high pressure when it is first bottled or canned. This high pressure environment increases the solubility of the CO2; therefore, more carbon dioxide is dissolved into the soda. When the pressure is released, when the soda is opened, the pressure in the container equals the pressure of the atmosphere, forcing the excess CO2 to come out of solution. (I can’t wait to talk about equilibrium constants!) What do you wash your clothes with? In Chemistry terms, laundry detergent is referred to as a surfactant. A surfactant or surface active agent, is a molecule that is amphiphilic which means that it has a water loving end (hydrophilic) and water fearing end (hydrophobic). These agents are wetting agents that lower the surface tension of a liquid, allowing easier spreading, and lower the interfacial tension between two liquids. Detergent is a manufactured water soluble surfactant that emulsifies oil and dirt; makes a suspension from one liquid into another where the first liquid does not dissolve in the second (kind of like oil and vinegar salad dressing). Soap is a naturally occurring detergent that emulsifies dirt. Acids and bases are electrolytes In solution acids are sour and bases are bitter Vinegar is an acid; Alum (which is used to make pickles) is a base Acids react with certain metals to make hydrogen gas Acids and bases neutralize each other In other words, they raise/lower the pH to a non-acid/non-base Acids and Bases can both destroy human tissue Naming Binary Acids o Acids are formed when a Hydrogen is added to an anion o Take the anion root, o Add a hydro- in front o Change the ending from –ide or -ate to –ic; or from –ite to -ous o Put them all together and add the word “acid” Naming Bases; Simply name the cation and anion There are 3 different types of acids and bases o Arrhenius o Brönsted-Lowry o Lewis Arrhenius Acids and Bases o An Arrhenius Acid is a substance that releases H+ ions in an aqueous solution o An Arrhenius Base is a substance that releases –OH in an aqueous solution Brönsted-Lowry Acids and Bases o A Brönsted-Lowry acid is any species that can donate a proton (H+ ion) to another species; a proton donor o A Brönsted-Lowry base is any speices that can accept a proton (H+ ion) from another species; a proton acceptor Amphiprotic: A compound that can be both a proton donor OR a proton acceptor in separate reactions is called amphiprotic or amphoteric Lewis Acids and Bases o A Lewis Acid is any species that can accept a pair of electrons from another species; electron pair acceptor o A Lewis Base is any species that can donate a pair of electrons from another species; electron pair donor Acid-Base Equilibria In a chemical reaction involving both acids and bases, dynamic equilibrium can still be achieved The strength of an acid depends on a number of factors, such as the properties of the solvent, the temperature, and the molecular structure of the acid. We compare the strengths of two acids, in the same solvent and at the same temperature. That way we can focus on the structure of the acid. Ionization Constants of Acids and Bases o HA(aq) + H2O(l) ↔ H3O+(aq) + A-(aq) o Keq= [H3O+][A-] / [HA][H2O] o Keq= [H3O+][A-] / [HA] pH + pOH = 14 o pH = -log10[H3O+] o pOH = -log10[OH-] Titration is a common laboratory method of quantitative/chemical analysis which can be used to determine the concentration of a known reactant o MV = MV o The titrant is usually the solution of known concentration that is delivered by a burette into a known quantity of the solution of unknown concentration o The titraver is the indicating solution Ionization Constants o Kw= [H3O+][OH-] o Kw = 1.0 x 10-14 Oxidation-Reduction: “Redox Reactions” Oxidation Loss of electrons Na Na+ + eReduction Gain of electrons Cl + e- ClOxidation Number (Oxidation State) The charge that an atom would have if the electrons in the bond were possessed entirely by the more electronegative element. Oxidation numbers serve as a bookkeeping tool used to keep track of electron movement. Assigning Oxidation Numbers o All pure elements and homogeneous molecules = 0 o Elements in group IA = +1 o Elements in group IIA = +2 o Ag+, Zn+2, Al+3 o In binary compounds the second element = anion charge o Oxygen is almost always = -2 o Hydrogen is almost always = +1 o The total charge of a compound is always = 0 Oxidizing/Reducing Agents To determine if a reaction is redox, determine the oxidation numbers for all elements. o The element that was oxidized is part of the reducing agent. o The element that was reduced is part of the oxidizing agent.





