Continuing Review Reporting Form
advertisement

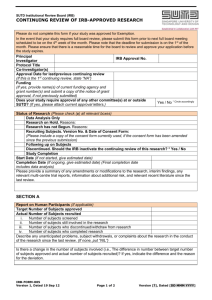
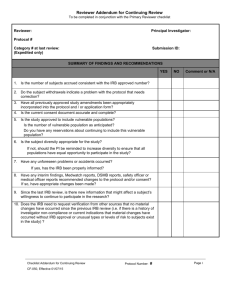
F-12 Continuing Review Reporting Form Please ensure that you complete this form within the appropriate time allotted for Continuing Review. Failure to provide this form at the designated time will cause approval for the site to expire. You may fax this document and any attachments to (740) 206-1601. 1. Study Information Protocol Number: Sponsor: Principal Investigator: Approval Expiration Date: 2. Study Information and Documents Required for Continuing Review Please provide the following information regarding the documents you are currently using at your site: Initial Protocol Version: ; Dated: Latest Amendment Version (if applicable): ; Dated: The Most Recent Consent Version: ; Dated: Please provide a copy of the first page of each template consent/assent you are currently using OR the most recently signed consent/assent with no subject identifiers; Number of consents: (includes the number of assents and consents) Current copy of the Principal Investigator’s Medical License Continuing Review (when applying for re-approval of the study at your site). Please select from the following options: Currently, participants are enrolled Currently, no participants have enrolled Enrollment is completed, however, the following activities are being conducted at the site: Participants are receiving study treatment/intervention Participants have completed study treatment/intervention but continue in follow-up activities Participant involvement is completed but renewal is requested for data analysis Study is closed and no active participants (Submit Close Out Reporting Form) Date the site anticipates to submit the close-out report: Unknown 3. Participant Enrollment Information a. Total Number of Active Participants b. Total Number of Participants Who Signed the Consent Form: c. Total Number of Screen Failures (did not meet inclusion/exclusion criteria): d. Number of Participants Randomized, THEN withdrew*, if applicable: e. Number of Participants Who Completed the Study: f. Authorized Number of Participants for Enrollment by the Sponsor: g. Total Project Enrollment at the Site: *Reasons for participant withdrawal (if any – please be specific): Continuing Review Reporting Form Version 6, dated March 10, 2011 Page 1 of 4 Protocol Number: Principal Investigator: 4. Research Participant Demographics – Please provide the number of each demographic participating in the research at your site Gender: Origin: : # of Males #: Caucasian #: African Descent #: Asian : # of Females #: Middle Eastern #: Pacific Islander #: Hispanic #: Native American #: Other Age: #: 0-17 #: 18 – 64 #: 65+ yrs. 5. Participant Safety – when completing this section, please provide data from either the last continuing review or a total for the study Since last Review a. Total Number of Serious Adverse Events: b. Total Number of Unanticipated Problems: c. Total Number of Protocol Deviations: d. Total Number of Adverse Events that did not require reporting to Harrison IRB*: e. Total Number of minor Protocol Deviations that did not require reporting to Harrison IRB*: f. Total Number of Research-Related Injuries**: g. Total Number of Participants requesting compensation for injury**: h. Have there been any problems at your site regarding the confidentiality of participant data, or incidents that may have compromised the privacy interests of research participants? If yes, please explain: Study Total Yes No Yes No Yes No Yes No i. Have there been any complaints about the research? If yes, please explain: *In your opinion, do the total number of adverse events and protocol deviations indicate that changes to the research plan and/or informed consent form be made? Yes No (if yes, please provide justification for the suggested changes) **Please attach a report regarding research-related injuries and participants requesting compensation for injury if you have not previously submitted this information to Harrison IRB. Attached report N/A 6. Participant Recruitment Information a. Has your site used or is using advertising materials that have been previously approved by Harrison IRB? b. Are any non-English speaking participants enrolled at your site for this study? c. If yes, are you using the informed consent translation that was approved by Harrison IRB? If no, please submit a copy of the consent document you are using. Continuing Review Reporting Form Version 6, dated March 10, 2011 Yes Yes Yes No No No Page 2 of 4 Protocol Number: Principal Investigator: 7. Modifications and Changes a. Have there been any changes in community attitudes or state laws since your last approval? If yes, please explain: Yes No Yes No Yes No Yes No Yes No f. Have there been any site specific changes since your last IRB review? (i.e., change in address, change in PI) If yes, please explain: Yes No g. Has anything happened at your site, not addressed in this report, that should be reported to Harrison IRB? If yes, please explain: Yes No b. Have there been any changes OR are you requesting any changes in participant population, recruitment, study procedures or consent procedures at your site? If yes, please explain: c. Have there been any changes in conflict of interest disclosure information per 21 CFR 54 since initial submission of your site? If yes, please explain: d. Has your site been audited by the FDA since your approval/last continuing review report? If yes, please submit a copy of the report, if applicable e. Have any vulnerable subject populations been recruited for this study that was not reported at the time of initial review by Harrison IRB? If yes, please provide details: 8. Additional Information – Please submit the following information, if available a. Any relevant recent literature. List the literature: b. Any interim findings, including data safety monitoring reports c. Any relevant multi-center trial reports d. The investigator’s current risk-potential benefit assessment based on study results Continuing Review Reporting Form Version 6, dated March 10, 2011 Attached Not Available Not Applicable Attached Not Available Not Applicable Attached Not Available Not Applicable Attached Not Available Not Applicable Page 3 of 4 Protocol Number: Principal Investigator: By signing this form, the Principal Investigator certifies that he/she has disclosed to Harrison IRB all relevant information concerning adverse events or other issues that might affect the risk-to-benefit analysis of this study. Principal Investigator Name: Principal Investigator Signature: Continuing Review Reporting Form Version 6, dated March 10, 2011 Date: Page 4 of 4