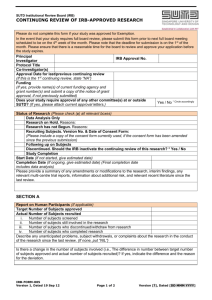
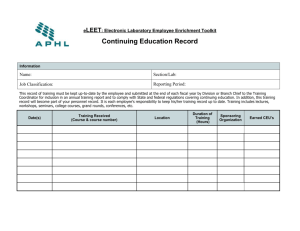
Form 7 - Continuing Review Application
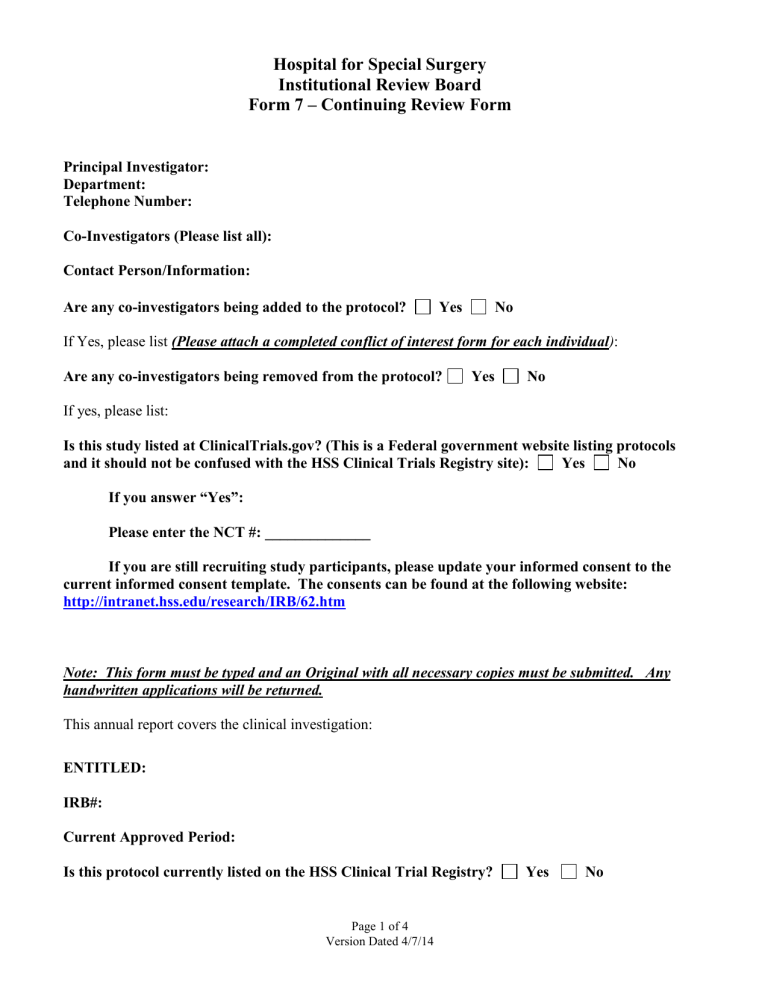
Hospital for Special Surgery
Institutional Review Board
Form 7 – Continuing Review Form
Principal Investigator:
Department:
Telephone Number:
Co-Investigators (Please list all):
Contact Person/Information:
Are any co-investigators being added to the protocol? Yes No
If Yes, please list (Please attach a completed conflict of interest form for each individual) :
Are any co-investigators being removed from the protocol? Yes No
If yes, please list:
Is this study listed at ClinicalTrials.gov? (This is a Federal government website listing protocols and it should not be confused with the HSS Clinical Trials Registry site): Yes No
If you answer “Yes”:
Please enter the NCT #: ______________
If you are still recruiting study participants, please update your informed consent to the current informed consent template. The consents can be found at the following website: http://intranet.hss.edu/research/IRB/62.htm
Note: This form must be typed and an Original with all necessary copies must be submitted. Any
handwritten applications will be returned.
This annual report covers the clinical investigation:
ENTITLED:
IRB#:
Current Approved Period:
Is this protocol currently listed on the HSS Clinical Trial Registry? Yes No
Page 1 of 4
Version Dated 4/7/14
Hospital for Special Surgery
Institutional Review Board
Form 7 – Continuing Review Form
1.
2.
3.
Sponsoring Agency:
Special Population
Minors Mentally disabled Pregnant woman
Prisoners None of the preceding
Status of Research ( please read options carefully )
Abortuses/Fetuses
Active – Open for enrollment (See your memo for submission copies)
Funding pending ( See your memo for submission copies)
Terminated – Provide reason for termination ( Submit 1 original and 1 copy)
No further enrollment (If study related interventions continue, see memo for submission copies)
Research is permanently closed to enrollment AND r esearch-related interventions are completed
AND active for ONLY long-term follow up ( Submit 1 original and 2 copies- no consent form
necessary)
No subjects enrolled AND no additional risks have been identified ( Submit 1 original and 2 copies)
Remaining research activities limited to data analysis (Submit 1 original and 2 copies – no consent
form is necessary)
Completed – (Submit 1 original and 1 copy; attach a final report (i.e. publication or summary of
findings); no consent form is necessary)
Note:
Please resubmit requests for HIPAA waivers where applicable. Submit a newly dated and signed Financial Interest Disclosure Form for all study team members listed on the protocol.
4. Provide the number of subjects enrolled in the study to date according to the following categories.
Female
American
Indian/Alaskan
Male
Unknown
Asian/Pacific
Islander
Black, Not
Hispanic
Hispanic White Unknown TOTAL
TOTAL
Page 2 of 4
Version Dated 4/7/14
Hospital for Special Surgery
Institutional Review Board
Form 7 – Continuing Review Form
Has the total enrollment exceeded the approved estimated number of subjects/specimens? No Yes
If yes, please attach a request to increase the number of subjects in the form of a memo to the IRB.
Approximately how many subjects have withdrawn during the study to date?
Please explain WHY these subjects have withdrawn. Please use as much detail as possible.
Have any adverse events or side effects occurred:
During the course of this study? No Yes
Of these, have any occurred since the last continuing review? No Yes
If yes, describe in detail, on an additional page if necessary, the cause of the adverse event or side effects and its resolution.
Have any unanticipated problems occurred during the course of this study?
If yes, please describe in detail below. Use an additional page if necessary.
No Yes
Since the last reporting period, have any significant findings developed which may relate to the subject’s willingness to continue? No Yes
If yes, please describe the findings and indicate whether subjects currently enrolled have been informed.
Page 3 of 4
Version Dated 4/7/14
Hospital for Special Surgery
Institutional Review Board
Form 7 – Continuing Review Form
Describe your progress to date and any results or conclusions that may be drawn from the data collected
(if needed, please attach on a separate sheet of paper).
Below, please describe recent literature findings that are relevant to this study. Use additional pages as necessary.
Please summarize any other information you feel is relevant for the board to consider when re-reviewing your continuing review submission.
Is this a Continuing Review for a Registry? No Yes
If yes, please list all approved requests for data since the last review and indicate those that have been presented or published (if needed, please attach on a separate sheet of paper).
I certify that I have on file, copies of all signed consent forms for the above referenced study.
Principal Investigator ___________________________ _________
Signature Date
RESEARCH Service Chief ___________________________ _________
Signature Date
Page 4 of 4
Version Dated 4/7/14