Worksheet Reactions of Alkal and Halo with ANS
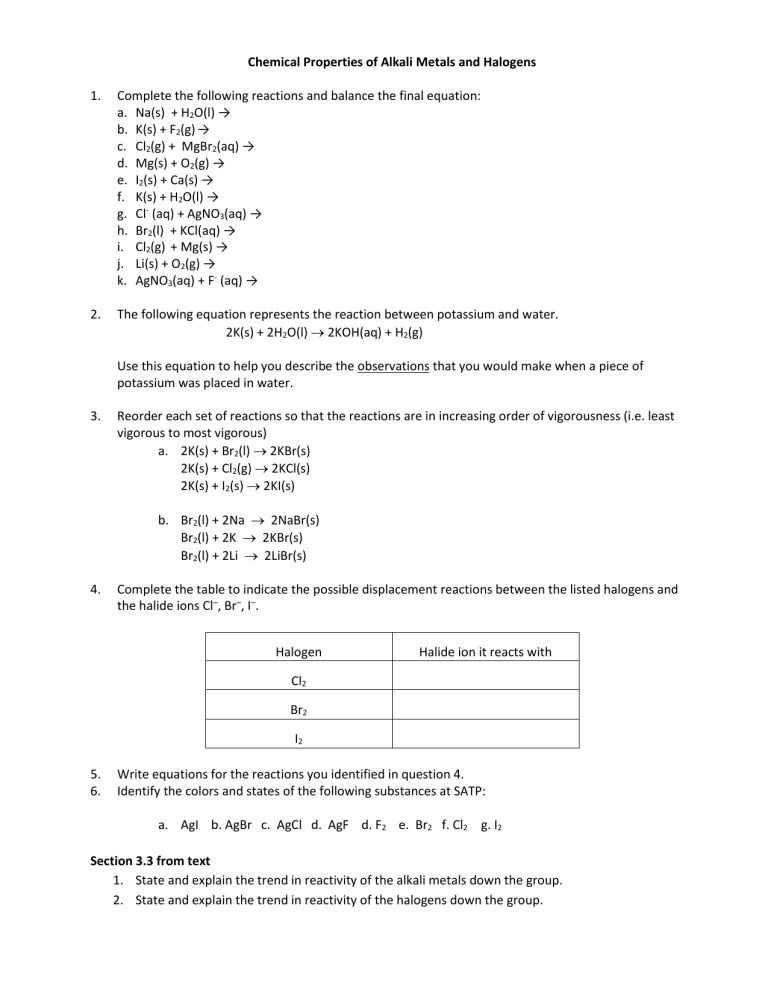
Chemical Properties of Alkali Metals and Halogens
1.
Complete the following reactions and balance the final equation: a.
Na(s) + H
2
O(l) → b.
K(s) + F
2
(g) → c.
Cl
2
(g) + MgBr
2
(aq) → d.
Mg(s) + O
2
(g) → e.
I
2
(s) + Ca(s) → f.
K(s) + H
2
O(l) → g.
Cl (aq) + AgNO
3
(aq) → h.
Br
2
(l) + KCl(aq) → i.
Cl
2
(g) + Mg(s) → j.
Li(s) + O
2
(g) → k.
AgNO
3
(aq) + F (aq) →
2.
The following equation represents the reaction between potassium and water.
2K(s) + 2H
2
O(l)
2KOH(aq) + H
2
(g)
Use this equation to help you describe the observations that you would make when a piece of potassium was placed in water.
3.
Reorder each set of reactions so that the reactions are in increasing order of vigorousness (i.e. least vigorous to most vigorous) a.
2K(s) + Br
2
(l)
2KBr(s)
2K(s) + Cl
2
(g)
2KCl(s)
2K(s) + I
2
(s)
2KI(s) b.
Br
2
(l) + 2Na
2NaBr(s)
Br
2
(l) + 2K
2KBr(s)
Br
2
(l) + 2Li
2LiBr(s)
4.
Complete the table to indicate the possible displacement reactions between the listed halogens and the halide ions Cl – , Br – , I – .
Halogen
Cl
2
Halide ion it reacts with
Br
I
2
2
5.
Write equations for the reactions you identified in question 4.
6.
Identify the colors and states of the following substances at SATP: a.
AgI b. AgBr c. AgCl d. AgF d. F
2
e. Br
2 f. Cl
2
g. I
2
Section 3.3 from text
1.
State and explain the trend in reactivity of the alkali metals down the group.
2.
State and explain the trend in reactivity of the halogens down the group.
3.
a. State the balanced chemical equation for the reaction of lithium with water, including states. b.
State the sort of solution that is produced in the reaction in part a. c.
Explain how you could show that this is the type of solution produced.
4.
a. List the possible halide ions with which chlorine is able to react. b. List the possible halide ions with which iodine is able to react.
5.
Write balanced chemical equations for the reactions of chlorine with the halide ions, Br− and I−.
6 Compare the reactions of: a. lithium and sodium with water b. chlorine and bromine gas with sodium c . fluorine and chlorine gas with bromide ions d. sodium and potassium with bromine gas.
ANSWERS
1.
Complete the following reactions and balance the final equation: a.
2Na(s) + 2H
2
O(l) → 2NaOH (aq) + H
2
(g) b.
2K(s) + F
2
(g) → no rxn c.
Cl
2
(g) + MgBr
2
(aq) → Br
2
(l) + MgCl
2
(aq) d.
2Mg(s) + O
2
(g) → 2MgO(s) e.
I
2
(s) + Ca(s) → CaI
2
(s) f.
2K(s) + 2H
2
O(l) → 2KOH(aq) + H
2
(aq) g.
Cl (aq) + AgNO
3
(aq) → AgCl(s) + NO
3
(aq) h.
Br
2
(l) + KCl(aq) → no reaction i.
Cl
2
(g) + Mg(s) → MgCl
2
(s) j.
4 Li(s) + O
2
(g) → 2Li
2
O(s) k.
AgNO
3
(aq) + F (aq) → AgF(s) + NO
3
(aq)
2.
The potassium would melt and ‘whiz’ around on the surface of the water, bursting into a violet flame.
3. a 2K(s) + I
2
(s)
2KI(s)
2K(s) + Br
2
(l)
2KBr(s)
2K(s) + Cl
2
(g)
2KCl(s) b Br
2
(l) + 2Li
2LiBr(s)
Br
2
(l) + 2Na
2NaBr(s)
Br
2
(l) + 2K
2KBr(s)
4.
Increasing vigorousness of reaction
Increasing vigorousness of reaction
Halogen Halide ion it reacts with
Cl
Br
2
2
Br – , I –
I –
I
2
5. Cl
2
(g) + 2Br– (aq) → Br
2
(aq) + 2Cl– (aq)
Cl
2
(g) + 2I– (aq) → I
2
(aq) + 2Cl– (aq)
Br
2
(g) + 2I– (aq) → I
2
(aq) + 2Br– (aq)
–
6. a. yellow solid b. e. yellow gas f.
Section 3.3 from Text cream solid red liquid c. g. white solid green gas d. h. doesn’t form purplish black solid
1 All alkali metals have one valence electron, but the reactivity of the alkali metals increases down the group. For each element down the group, there is an extra electron shell. The further the valence shell is from the nucleus, the more easily the valence electron is lost in a reaction.
2 The halogens become less reactive down the group. The larger the halogen atom, the smaller is its ability to gain electrons, as they are further from the nucleus, and thus there is less attraction.
3 a 2Li(s) + 2H
2
O(l)
2LiOH(aq) + H
2
(g)
b. An alkaline solution is produced.
c. Add phenolphthalein (or other pH indicator) to the water before the reaction; the indicator will turn pink due to the presence of LiOH.
4 a Br – and I –
b none
5 Cl
2
(g) + 2Br – (aq)
2Cl – (aq) + Br
2
(aq)
Cl
2
(g) + 2I – (aq)
2Cl – (aq) + I
2
(aq)
6 a Sodium will react more vigorously with water than lithium.
b Chlorine gas will react more vigorously with sodium than bromine gas.
c Fluorine gas will react more vigorously with bromide ions than chlorine gas.
d Potassium will react more vigorously with bromine gas than sodium.