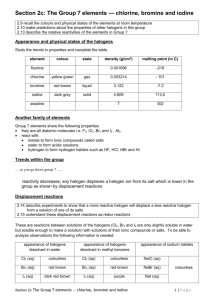
A. Teacher Demo Halogens...printou
advertisement

Halogen Lab Chemistry Name ________________________________ per __________________ Purpose : to investigate the reactions between Halogens atoms and ions and be able to predict reactions and write balance equations Equipment : Small test tubes, stoppers, brushes and rack. Droppers with chloride, promide and iodide ion. Dropper with Chlorine water and SOLVENT. The solvent will dissolve molecules of Bromine or iodine out of water solution. Procedure : 1. Confirm the fact that Chlorine will react with bromide and iodide ions. Do the reactions and describe what happens in the water. Add the SOLVENT to confirm your results. Write a balance equation for the reaction. After this is done then react Bromine with iodide ion and observe the results. You will have to figure out a way to make your own Br2 for any reactions you may want to perform with it. Here is an Example of one reaction : _____ Cl2 (aq) + _____ Br -1 (aq) ---------> __________ + ____________ _____ describe – ___________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 2. “ Like dissolves like “ is a statement in chemistry that describes the fact that water which has definite (-) and (+) ends easily dissolves ions and other strongly polar molecules. Whereas, nonpolar solvents like gasoline tend to dissolve other nonpolar molecules like grease. a. Is the SOLVENT more or less dense than water?__________________ b. Does the SOLVENT mix with water? _______________________ c. Is the SOLVENT more likely, then, to be POLAR or NONPOLAR? ___________________ d. Ions are more likely to dissolve in WATER or SOLVENT?____________________ e. Molecules of chlorine, bromine, iodine and fluorine are not polar. These molecules will dissolve best in WATER or SOLVENT? ______________________________ f. Molecules of Iodine tend to leave the water when SOLVENT is added and the mixture is shaken . Explain : ________________________________________________________________________________________________ Finish balancing the following equations. If no reaction takes place write NR. a. F2 (aq) + Cl -(aq)-----> b. Cl 2 (aq) + F - (aq) ------> BALANCE!! c. F2 (aq) + I -1 ---------> d. Br2 + I -1 -----------> e. I2 + Br -1 -----------> f. F2 + Br -1 ------------> g. Cl -1 + Br - ----------> h. Cl 2 + Br2 -----------> i. Cl -1 + F2 -------------> j. I -1 + Cl2 ------------> If the halogen ion is written in compound for ... like NaCl instead of Cl -1 the balancing is the same.... sodium ju becomes a spectator ion. Example : 2 Na Br (aq) + 1 Cl2 ----------> 2 NaCl (aq) + OR 2Na +1 (aq) + 2 Br -1 (aq) + 1 Cl2 (aq) ------------> 2 Na +1 (aq) + 2 Cl -1 (aq) + 1 Br2 (aq) 1 Br2 (aq) k. ___ K Br (aq) + ___ Cl2 (aq) ----------> l. ____ F2 (aq) + ___ CsCl (aq) ----------> m.____ NaCl (aq) + ____ K I (aq) -----------> n.____ Na Br (aq) + ____ Cl2 (aq) ----------> o. ____ Li I (aq) + ____ Br2 (aq) -----------> p.____ Ba I 2 (aq) + ____ Cl 2 (aq) ------------> _____NaCl (aq) + ____ Cl2 (aq) -----------> 4. Here is an example of what happens when a halogen molecule reacts with water 3 I2 + 3 H2 O <---------> 5I - + 6 + + IO3In this reaction all 4 substances are found in the solution because it does not go to completion to the right Remember that Universal Indicator color tells approximate hydrogen ion concentration. Devise a chemi test to show that a halogen molecule will produce some hydrogen ions in its reaction with water. Describe below write out as many balance chemical equations as possible. Get some U.I. and do it!! Extra Credit