chem ethanol 2007

THE EFFECT OF DIFFERENT DILUTION RATES ON THE PRODUCTION OF
ETHANOL IN A CHEMOSTAT
Edwin Chiu, Gemma Fitzpatrick, Philipp Guthrod, Julius Kuah, Bastian Piltz, and
Ewe Xjin Lim.
Industrial Bioprocessing and Bioremediation, Murdoch University
Abstract
A chemostat was run with Saccharomyces cerevisiae under anaerobic conditions using “Pure Super Turbo” yeast in order to produce ethanol. The conditions of the bioreactor, which were kept constant, was the temperature at 30 o C, and an rpm of 4 units. The main constituent in the feed vessel was D-glucose at 75g/L, a much higher amount tested than in previous studies. Two different dilution rates (D) of 0.015h-1 and 0.075h-1 were also tested over 4 days and 7 days respectively. The lower dilution rate produced an ethanol concentration of 26.36g/L in 4 days, and the higher dilution rate almost doubled the ethanol concentration produced to 52.62g/L. Previous reports have shown the optimal dilution rate to be 0.0625h-1, but our increased dilution rate gave an even larger concentration of ethanol produced by the yeast. The maximum productivity at D=0.075 was 0.02175g/L/h. The maximum yield at D=0.075h-1 was
0.0065g/L/h. Both the yield and productivity were higher at this dilution rate.
Introduction
The art of using microbes to produce various fermentation products has been known for many centuries. The first evidence for this use is over 9000 years old, where
Chinese used alcoholic beverages as intoxicating ingredients(Roach, 2005). The further development of this technique led to the process of beer brewing and wine production as well as bread and cheese manufacturing. All processes have in common that they use a carbohydrate source (like wheat, rye, corn, potatoes) which is converted into ethanol and carbon dioxide under anaerobic conditions. Traditionally these fermentation products are made in batch culture and are not run continuously.
But the enlarged industrial interest in bio ethanol as a alternative energy source led to large scale production preferred in batch culture systems because they have until now greater advantages compared to continuous culture systems like a chemostat.
The main problem is the maintenance of sterility during a chemostat run.
Contamination from outside as well as from inside could destroy a whole culture.
Outside contamination is the result of hardly achievable sterilisation of the continuous system, while inside contamination arises from the growth of respiratory deficient mutants (RDM). The time needed for a chemostat to reach a steady-state of biomass, makes it even more uneconomic for industrial large scale purposes. But after all chemostats have advantages over batch systems because they will have higher rates of productivity, constant product formation, continuous output, the ability to monitor and change several parameters that increase productivity and reduce labour force (Cord-
Ruwisch, 1997).
Ethanol is a fuel superior to gasoline as it is a more clean combuster, and can be produced from renewable sources. Today’s technological expertise at producing bioethanol as fuel is not advanced enough as to make it an economically and ecologically feasible process, mainly because of the large amount of energy needed for the distillation and thereby purification of the ethanol.
The aim of industrial bioethanol production is to have a positive energy yield under conditions that can maintained continuously and that do not affect the environment or food supply in a negative way. Therefore substrates that are no longer used for food must be used.
We used a special brewing strain of Saccharomyces cerevisae called Super Turbo
Yeast which was selected for increased ethanol production. We fed them with glucose which was converted anaerobically according to the following biochemical reaction(Becker and Deamer, 1991):
Glucose + 2ADP + 2Pi
2 Ethanol + 2ATP + 2H
2
O +2CO2
The electron-carbon flow and the steps of conversion can be seen in the following figure:
Figure 1 Electron/Carbon flow in ethanol formation (
Ralf Cord-Ruwisch)
The aim of our experiment was the continuous anaerobic fermentation of D-glucose into ethanol using the yeast S. cerevisiae under two different dilution rates in order to optimise productivity.
Materials and Methods
EQUIPMENT
Schott Bottle (2L)-for feed vessel, harvest vessel and
Reactor
Retort Stand(+ clamps)
QUANTITY
3
1
1 Automatic Aquarium Heater (Type RS 25), Blutherm set at 30 o C
Thermometer
Stainless Steel Pot (as water bath)
1
1
2 Styrofoam Boxes (holding ice which was packed around the feed vessel and harvest vessel)
Gas Trap for CO
2
measurements (attached to reactor)involved a 1L cylinder with a 250mL cylinder turned upside down inside the 1L one, filled with water
1
2 10mL Syringe (one to measure product each dayattached to harvest vessel tubing, and one connected directly to reactor)
Pressure outlet (attached to reactor)
Tubing clamps(plastic or wire)-in order to keep system sealed + anaerobic
Terumo needle 25
G x 3/4” (0.5 x 19mm)-part of dripper system leading into reactor.
Flea
Flea catcher
IEC magnetic stirring plate (that reactor is placed upon)
Timer (LT48W)
Chemaster Pump (DEMA Australia), 240V 50HZ
0.5L plastic container (to raise pump off bench to help shorten tubing)
Silicone tubing (approx 3mm wide)
Rubber bungs with holes for tubing
Parafilm(to wrap around the mouth of the reactor vessel where the rubber bung sits with the tubing flowing in and around the needle)
3 way connector(for tubing flowing around the harvest vessel, the collection syringe, and the outflow of pump)
1 several
2
1
1
1
1
1
1
Approx 1m
4
1
Set up of the second and final chemostat
Silicon tubing
Inflow of glucose needle
Outflow of ethanol
Timer
Feed
Vessel (2L)
IN
Pump
OUT
Box to raise pump up
Heater
Figure 2. Chemostat design
Reactor
Vessel(2 L)
500mL
Magnetic Flea
Magnetic Stirrer
(rpm=4 Units)
9
10mL syringe
Thermometer
(at 30 O C)
Retort Stand
CO
2 measurements
Pressure outlet
10mL sampling syringe
3 way connector
Sealed
Styrofoam box filled with ice
Harvest Vessel
(2L)
MEDIA
D-glucose
Yeast extract
NH
4
Cl
MgSO
4
.H
2
0
CaCl
2
H
2
0 g/L
75
8.5
1.3
0.11
0.66
Make up to 1L
The quantities of constituents in the media were chosen based on previous reports(Gibbs et al ., 1998) due to their results of a good ethanol production rate.
Previous groups did not add as much glucose to their media due to their reactor vessel already containing glucose in the form of apple juice for example. The amount of glucose used was based on trying to increase the EtOH production further.
Inoculum
Was prepared using Alcotec “24 hour Pure Turbo Super Yeast” (
S.cerevisiae
) obtained from Murdoch University. 7.031g was provided, all of which was added to the reactor. This yeast was chosen due to it being a high alcohol and temperaturetolerant dual-function yeast, complete with needed nutrients, and the ability to yield up to 14% alcohol in 24 hours.
Media
The media was always autoclaved as well as the tubing leading from the feed vessel to the pump to prevent contamination. The media was replaced daily once the dilution rate was increased to 0.075h
-1
but initially at 0.015h
-1
it was replaced approximately twice weekly. Ice was replaced daily in Styrofoam boxes.
Batch Culture
Initially a batch culture was set up with 7.031g of the super turbo yeast and 14.22g of glucose made up to 500mL with water. This was run for 4 days to make sure the yeast grew and then the setup was changed to a chemostat.
When any problems resulted with the chemostat, such as contamination, we always reverted to a batch culture before setting up a new chemostat.
Chemostat setup
Silicon tubing was inserted as opposed to plastic tubing so it can be autoclaved, and is more sturdy. The tubing was made as short as possible so there was less chance of contamination and the pumping was more effective. Tubing was autoclaved wrapped in aluminium foil.
Reactor Vessel
The vessel was maintained at 30 o
C through heating the water it was sitting in with an aquarium heater. A magnetic stirrer and a magnetic flea were used to ensure that the reactor was stirred continuously and evenly. The rpm was 4 units(or the 4 th notch on the control).
After growth in batch mode for 4 days, the reactor vessel was connected to the feed vessel, the sample syringe, and the gas cylinder for CO
2 measurements. All attachments to all vessels were made as airtight as possible with wire or plastic clamps in order to keep the system anaerobic. This was also the reasoning behind the rubber bungs and the parafilm around the mouth of the reactor vessel.
2
3
4
5
Harvest vessel
A pressure outlet was fitted to the rubber bung in the harvest vessel to allow air to enter and leave the vessel, keeping air pressure constant. It also aided in preventing contaminants from the air entering the vessel.
Ethanol:
Ethanol Concentration was analysed via the All Tech All scribe 3300 Gas
Chromatography (GC) machine. The samples, collected as often as possible, at approximately the same time each day(12.30pm) from the harvest vessel with a 10mL syringe were stored in the freezer in GC bottles until needed, to prevent further fermentation and ethanol evaporation. When the samples have been defrosted ready for use, it is imperative to be as quick as possible with the analysis for this reason. A syringe designed to be inserted into the GC was used to take a sample from the head space of the GC bottle, and this was inserted into the machine. The amount of EtOH produced can be quantified by comparing the peak heights obtained with peaks belonging to standards of known ethanol concentration.
Biomass:
Biomass was calculated by measuring Optical Density (OD) at 540nm using UV mini
1240: UV-Vis spectrophotometer (Shimadzu).
Calibration of Pump and Determining Medium Flow Rate
After the chemostat was assembled, the flow rate was determined by pumping water through the chemostat. The reactor was filled with 500mL of water, and the feed bottle was filled with 1 L of water. The outflow of the chemostat was sent to a measuring cylinder. This process was timed using a digital stopwatch.
After 1 minute the chemostat was switched off, and the volume of water collected in the measuring cylinder indicated the amount of liquid flowing through the chemostat in 1 minute. The process was repeated three times, and the average of the flow rate per minute was calculated. The trial was repeated to test all the pump settings to determine the flow rates.
Pump Setting Flow Rate (mL
1
/ min)
4.5
Flow Rate (L / h)
0.270
7
10.5
15
21
0.420
0.630
0.900
1.26
6
7
8
9
10
22.5
26
31
32
32
Connecting Timer to the pump:
1.35
1.56
1.86
1.92
1.92
The timer was connected to the pump to ensure a continuous feed to the reactor. It also assisted in slowing the flow rate down much lower than would be possible by pump settings alone. This allows the medium flow rate of a pump setting to be altered by a fraction, based on the on / off intervals that can be programmed. The fractional on / off interval can be calculated by dividing the desired (D) by the (D) of the pump setting used. For example, if a dilution rate half of the lowest pump setting is desired, the timer can be set to be on for 30 seconds and off for 30 seconds.
For D=0.015 the timer was set to an on-interval of 1.2s/min and correspondingly for
7.2s/min to reach D=0.075.
Setting up of Chemostat:
Ice was packed around the harvest vessel to prevent further fermentation(which would effect GC and biomass readings), and the feed vessel was also surrounded by ice to prevent bacterial growth. The dripper system seen flowing into the main reactor was designed to prevent back contamination into the substrate and partially controlling the flow rate.
Changes in setup
Initially the setup running into the reactor vessel was 2 syringes connected by 2 needles and rubber bungs( as was the setup in Pittman et al ., 2000), but this was found to be unnecessary, and when it came to testing the second dilution rate, this was changed to 1 syringe, 1 rubber bung, and 1 needle setup 1 setup 2
Figure 3: changes in setup between chemostats
Results
Ethanol production
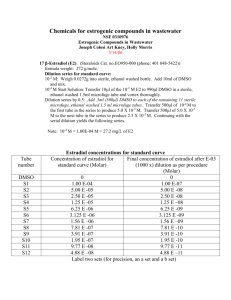
Table 1: Concentrations of ethanol standards used to determine the ethanol produced in the bioreactor over a period of time.
Concentration of ethanol g/L
GC
Peaks
Baseline: 20
Peaks-20
2
5
10
20
30
40
58.75
74.50
90.00
132.50
215.00
341.50
38.75
54.50
70.00
112.50
195.00
321.50
GC peaks Vs Conc. of Ethanol (g/L)
Standards
y = 7.0319x + 6.6402
400
350
300
250
200
150
100
50
0
0 10 20 30 40
Conc. of Ethanol (g/L)
50
Figure 4: Concentration of Ethanol Standards used to compare the concentration of ethanol produced from the bioreactor.
60
Concentraion of Ethanol(g/L) Vs Time (hours)
60.00
50.00
40.00
30.00
20.00
10.00
Dilution rate= 0.015 h-1
Dilution rate= 0.075 h-1
0.00
0 50 100 150 200
Time (hours)
Figure 5: Chemostat 1, the concentration of ethanol produced running at a dilution rate of 0.015h
-1 from the bioreactor over 4 days based on the standard curve obtained in Figure 1.
At D=0.015h-1 the ethanol concentration is maximal at 48(2 days), after which time it drops and then rises again towards day 4.
Biomass Absorbance
Standard Curve of yeast biomass (g/L) y = 1.7048x + 0.0006
1.2
1
0.8
0.6
0.4
1.8
1.6
1.4
0.2
0
0 0.2
0.4
0.6
Yeast Concentration (g/L)
0.8
1 1.2
Figure 6: Chemostat 1, the production of biomass based on the absorbance reading at an optical density of 540nm using a dilution rate of 0.015h
-1 from the bioreactor over 4 days.
Biomass concentration (g/L) Vs time (hours)
0.35
0.3
0.25
0.2
0.15
0.1
Dilution rate= 0.015 h-1
Dilution rate= 0.075 h-1
0.05
0
0 20 40 60 80 100 120 140 160 180 time (hours)
Figure 7: Chemostat 2, the concentration of biomass based on the absorbance at an optical density of 540nm using a dilution rate of 0.075h
-1 present in the bioreactor within 7 days.
The maximum biomass concentration is reached earlier with the lower dilution rate, after 50 hours as opposed to 150hours at a D of 0.075h-1. pH readings pH Vs Time (hrs)
Chemostat 1
Dilution rate: 0.015 h
-1
3.45
3.4
3.35
3.3
3.25
3.2
3.15
3.1
3.05
3
0 20 40 60 80 100 120
Time (hrs)
Figure 8: Chemostat 1, the pH changes within 4 days of the bioreactor running at a dilution rate of 0.015h
-1 .
Very variable pH results in chemostat , with the maximum being 3.4. This fell to lower levels than the second chemostat. Keeping the pH at approximately
3.5 would be preferable.
50
40
30
20
70
60 pH Vs Time (hrs)
Chemostat 2
Dilution rate: 0.075 h
-1
5
4.5
4
3.5
3
2.5
2
1.5
1
0.5
0
0 50 100 150 200
Time (hrs)
Figure 9: Chemostat 2, the pH changes of the bioreactor within the 7 days running at a dilution rate of 0.075h
-1 .
The pH in the second chemostat was more constant.
Glucose remaining (gS)
Glucose remaining (g/L) Vs Time (hours)
Dilution rate= 0.015 h-1
Dilution rate= 0.075 h-1
10
0
0 20 40 60 80 100 120 140 160 180
Time (hours)
Figure 10: Graph of amount of Glucose left over in the bioreactor of the 2 different dilution rates with respect to time.
By comparing the concentration of glucose remaining in the feed vessel at the two dilution rates, it is seen that D= 0.075 h
-1
used up more glucose than D=0.015h
-1
.
Productivity(g/L/h) Vs time(hours)
0.025
0.02
0.015
0.01
Dilution rate= 0.015 h-1
Dilution rate= 0.075 h-1
0.005
0
0 20 40 60 80 100 120 140 160 180 time(hours)
Figure 11: Ethanol productivity seen in the 2 different dilution rates over time.
The productivity at D=0.075h-1 resulted in an average of 0.02g/L/h, which is higher than the productivity at D=0.015h-1 which resulted in an average of 0.0025g/L/h. The maximal productivity of 0.022g/L/h at D=0.075h-1 occurs at the same point in time as the maximal biomass concentration (Figure7).
Yield product (g/gS) Vs Time (hour)
0.007
0.006
0.005
0.004
0.003
0.002
0.001
Dilution rate= 0.015 h-1
Dilution rate= 0.075 h-1
0
0 50 100 150 200
Time (hour)
Figure 12: The product yield of the 2 different dilution rates over time.
At a higher dilution rate of 0.075h-1, the product yield was the highest at 0.0065g/L/h.
While the lower dilution rate of 0.015h-1 has a lower value of 0.0055g/L/h.
Discussion
In this experiment, two chemostats were designed to determine the maximum productivity of ethanol using super turbo yeast. The observations of the batch culture suggest that biomass of yeast greatly increased over time. Due to good biomass growth in the batch culture, we decided to run the culture as a chemostat. The results in Figure 4 and 5 indicate that the yeast in the bioreactor was producing sufficient amounts ethanol (double the concentration in the case of the increased dilution rate).
The lower dilution rate produced a maximum ethanol concentration of 26.36g/L in 4 days, and the higher dilution rate almost doubled the maximum ethanol concentration produced to 52.62g/L. This is because the higher flow made more substrate per time available to the yeast and therefore they could be more productive.
The fact that the ethanol concentration is highest on the last day (of the first chemostat measurements), means the maximum concentration over time is unknown, as the concentration may have kept rising if it had been left longer. The reason the chemostat 1 was stopped at day 4 was due to time constraints. The fact that the GC could be used only once several samples were collected meant that you can’t tell what the EtOH concentration is and whether to keep running the chemostat until the maximum concentration is reached.
The biomass concentration increased over time at both dilution rates, as seen in
Figure7. This corresponds to the rise in EtOH production over time( as there is more yeast present to convert glucose to ethanol). The fact that the maximum biomass concentration is reached much sooner at D=0.015 is due to the lower washout rate
(than at D=0.075), making it easier for the yeast to accumulate in the reactor over time in high concentrations. The maximum biomass concentration reached is very similar in both chemostats( regardless to the time period this happens over). The colour of the solution in the bioreactor became darker everyday, which was a macroscopic sign of increasing biomass concentration.
It was important to monitor the pH of the reactor, because when the pH drops it provides conditions that will favour another species. As seen in Figure 9 ,the pH in the second chemostat was more constant which may have resulted in higher concentrations of ethanol being produced(in addition to the fact that the dilution rate was higher for this chemostat). Keeping the pH at approximately 3.5(as in chemostat
2) would be preferable. This finding correlates to the literature where a pH between 3 and 3.8 is recommended during fermentation to maintain healthy yeast (Cantarelli and
Lanzarini, 1989).
By comparing the concentration of glucose remaining in the feed vessel over time, it appears that more glucose is used up at a dilution rate of 0.075h
-1 than 0.015h
-1 (Figure
10). This is what would be expected, because if the rate at which feed is flowing through to the reactor is higher, there will be less left in the feed vessel. These values were obtained using the stoichiometric equation for fermentation with respect to the amount of ethanol produced. Therefore, after the concentration of glucose was converted to moles, the moles were divided by 2.
The maximum productivity of 0.022g/L/h ethanol(Figure 11) at D=0.075h-1 occurs at the same point in time(6 days) as the maximal biomass concentration (Figure7). This shows a direct link to increased amounts of yeast leading to increased amounts of ethanol. There is no relationship between productivity and biomass at the lower dilution rate. This is another reason showing the unsuitability of a lower dilution rate.
At D= 0.075h-1 the product yield was the highest at 0.0065g/L/h, while the lower dilution rate of 0.015h-1 had a lower value of 0.0055g/L/h(see Figure 12).
Conversion to EtOH is not high at D=0.015, which can be seen from the glucose concentration being higher than the ethanol concentration (meaning that there is more substrate remaining than product, so it hasn’t been converted).
In the five weeks the chemostats were run, several problems were encountered. We experienced some difficulties on day 3 and day 4 of the second chemostat as high pressure built up in the bioreactor because the substrate was unable to flow into the bioreactor. This caused the flow rate to change over time, which also affected the outcome of the result. The chemostat setup of the dripper system was changed to fix the problem that had occurred.
In the second chemostat unexpected bubbles appeared in the bioreactor on day 3 and day 4. This may have occurred due to the substrate and tubes leading from the substrate to the pump not being autoclaved on day 3. However, this contamination did not appear to affect the production of ethanol(as seen in Figure11). High amounts of ethanol prevent most bacterial growth throughout the chemostat(ethanol flows throughout the tubing apart from the feed vessel to the pump). Furthermore, the substrate medium was incubated at approximately 4°C. The reason for the presence of bubbles in the bioreactor still remains unknown.
From previous reports, results showed the maximum dilution rate was 0.0625h-1.
However, our results in Figure 11 show that the maximum productivity of ethanol is higher than the previous years at D=0.075h-1. Furthermore, there is an increase in biomass over time. In this chemostat, the inflow and the outflow of the system had increased. This however, meant there were problems with substrate always running out at the higher dilution rate. There was build-up of yeast in the silicon tubing, but not to the extent that the fluids couldn’t run through it. As can be seen by the results of the high ethanol concentration measured in the samples, our aim was towards high productivity, not high conversion efficiency(seen by the fact that the substrate ran out so quickly each day).
Although our productivity of EtOH was higher than that of previous years, it does not mean this is the optimal productivity that could result from super turbo yeast at these conditions. Anything lower than D=0.015h-1 would be ineffective, but higher dilution rates than D=0.075h-1 may produce more EtOH (but be less conversion effective, and have higher washout). Further tests need to be conducted in order to find the maximum possible productivity and conversion efficiency from glucose to ethanol.
Had time permitted we would have liked to try feeding though a waste product such as banana peels(once the starch had been degraded to fermentable sugars with the correct enzymes) to make ethanol, and see how effective this would be.
Recommendations for future Chemostat projects
Read the other chemostat reports to have an overview. It is important to decide on the settings for the chemostat (i.e. dilution rate, composition of medium, etc.), and what to expect from them.
Decide on the methods of quantifying levels of Biomass, Substrate and
Product in the chemostat. Read up and research on the chosen methods of quantification, and understand how to carry out the quantification. Sampling and quantification must be able to be carried out in a consistent and regular basis, so as to obtain sufficient and accurate data for the calculation of other important data for the chemostat (i.e. productivity, efficiency of substrate conversion to product, growth rate, etc.)
For example, in our Chemostat project: o Quantification of product (Ethanol): Gas Chromatography was used.
How does it determine the amount of Ethanol present in the sample?
Amount of sample required? What standards are needed? o Determining the amount of biomass: For this project, biomass levels were determined by comparing the optical density of the sample against standards. Points to note: What absorbency and wavelength will be used? o Determining amount of substrate (glucose):
It is important to autoclave the substrate. This is to minimise loss of substrate due to premature metabolism by contaminants.
Keep the lengths of tubing as short and narrow as possible. This is to reduce the amount of friction the pump has to overcome at low dilution rates. This is especially important for the outflow tubing, since sedimentation of biomass can occur there. Try to keep the feed bottle, reactor vessel and the harvest bottle on the same elevation to minimise the length of tubing needed.
Determine the flow rate of the chemostat before running it. Based on the flow rate, calculate the rate of medium flowing into the reactor and the rate of outflow leaving the reactor. Ensure that sufficient medium is present in the feed bottle and the harvest bottle is large enough to contain the outflow required for the duration between changes.
For example, a flow rate of 50mL per hour will cause, 1,200 mL of feed to be used, and 1,200mL of outflow. If the feed bottle runs out of substrate, the chemostat (which is always in operation) will drain out the reactor over time without any fresh medium flowing in. conversely, the harvest bottle can overflow as well.
The harvest bottle must be able to contain a larger volume than the feed bottle.
This is to prevent the bottle from breaking when the harvest is preserved by freezing in a freezer. (Water expands when it freezes)
Try to incorporate a pH meter into the reaction vessel so that the pH of the medium can be monitored constantly and conveniently.
no need to use so many needles for dripper system as suggested in past reports
make sure you autoclave tubing from feed to pump, and your media otherwise you will get contamination and have to start again.
make sure everything is as airtight as possible to maintain anaerobic conditions
References:
Becker,W.M. and Deamer,D.W.(1991). The World of the Cell, The Benjamin and
Cummings publishing company, Redwood City.
Cantarelli, C., and Lanzarini, G. (1989) Biotechnology Applications In Beverage
Production. Elsevier Science Publishers Ltd., London.
Cord-Ruwisch,R.(2007) Industrial Bioprocessing and Bioremediation manual,
Murdoch University, Perth, W.A.
Gibbs,B., Humphris,S., and Krishnamurthy,P (1998). Affect of Medium Flow Rate on
EtOH Production in a Continuous Stirred Tank Bioreactor. Murdoch Industrial
Microbiology Research Group.
Pittman,A., Chami,H., Eckersly,B., and Khosrowashahi,N(2000) Anaerobic fermentation of Apple, Cherry and Blackcurrant Juice for Ethanol Production in a
CSTR. Murdoch Indutrsial Microbiology Research Group.
Roach, J. ( 2005) 9,000-Year-Old Beer Re-Created From Chinese Recipe. National
Geographic News .
Appendix
Table 1.1: Ethanol concentration of the batch culture of the bioreactor for 4 days before starting a chemostat system. hrs
Time
Day No.
Ethanol conc. g/L
0
24
96
0
1
4
12.54
11.03
13.21
The EtOH concentration remained relatively constant throughout the running of the batch culture.
Biomass Absorbance
Table 3: The absorbance at 540nm of the bioreactor as batch culture over 4 days to determine the biomass concentration.
Time Absorbance hrs
0
24
Day no.
0
1
OD:540nm
0.331
0.394
96 4 0.455
The batch culture shows a relatively constant absorbance and hence biomass concentration over time.