Mouse liver ChIP assay
advertisement
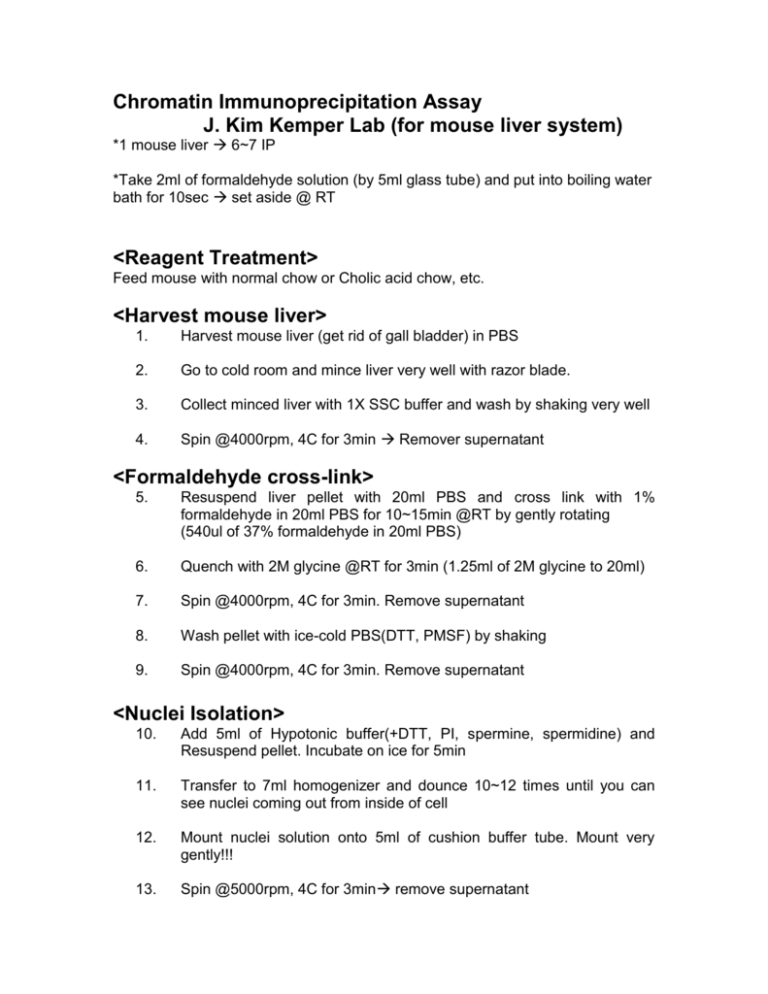
Chromatin Immunoprecipitation Assay J. Kim Kemper Lab (for mouse liver system) *1 mouse liver 6~7 IP *Take 2ml of formaldehyde solution (by 5ml glass tube) and put into boiling water bath for 10sec set aside @ RT <Reagent Treatment> Feed mouse with normal chow or Cholic acid chow, etc. <Harvest mouse liver> 1. Harvest mouse liver (get rid of gall bladder) in PBS 2. Go to cold room and mince liver very well with razor blade. 3. Collect minced liver with 1X SSC buffer and wash by shaking very well 4. Spin @4000rpm, 4C for 3min Remover supernatant <Formaldehyde cross-link> 5. Resuspend liver pellet with 20ml PBS and cross link with 1% formaldehyde in 20ml PBS for 10~15min @RT by gently rotating (540ul of 37% formaldehyde in 20ml PBS) 6. Quench with 2M glycine @RT for 3min (1.25ml of 2M glycine to 20ml) 7. Spin @4000rpm, 4C for 3min. Remove supernatant 8. Wash pellet with ice-cold PBS(DTT, PMSF) by shaking 9. Spin @4000rpm, 4C for 3min. Remove supernatant <Nuclei Isolation> 10. Add 5ml of Hypotonic buffer(+DTT, PI, spermine, spermidine) and Resuspend pellet. Incubate on ice for 5min 11. Transfer to 7ml homogenizer and dounce 10~12 times until you can see nuclei coming out from inside of cell 12. Mount nuclei solution onto 5ml of cushion buffer tube. Mount very gently!!! 13. Spin @5000rpm, 4C for 3min remove supernatant <Sonication & Preclearing> 14. Resuspend liver pellet with 1.8ml of SDS-lysis buffer (2mM EDTA, 50mM Tris pH8.0, final concentration of SDS should be 1%) divide into 4 tubes (each contains ~600ul) 15. Sonicate with 80% output for 40 sec until solution is going to be clear (10 sec X 4) 16. Spin @13000rpm, 4C for 10min 17. Collect supernatant and add 3-fold volume of dilution buffer (20mM TrisHCl pH8.0, 2mM EDTA, 200mM NaCl, 1% Triton X-100, 0.1% Nadeoxycholate) 18. Add (80ul Normal Goat Serum+200ul of 25% Protein A/G sepharose+ 5ug rabbit IgG) per mouse 19. Incubate @4C for 30min~1hour. <Immunoprecipitation > 20. Spin @8000rpm, 4C for 1min. Take supernatant only and combine all of them. 21. Save 1/10 for total chromatin. 22. Divide supernatant into IP tubes and add optimized amount of Ab O/N incubation @4C or at least more than 4hour incubation @4C. <Collect Immune complex> 23. Collect immune complex by adding 40ul of 25% protein A/G sepharose rotate @4C for 1~2hrs, no more than 2hours. <Wash and Elute> 24. Spin @8000rpm, 4C for 1min. Remove supernatant only 25. Wash protein A/G sepharose with TSE I once (+DTT, PI) rotate @4C for 5min centrifuge @8000rpm for 1min, remove supernatant. 26. Wash with TSE II once (+DTT, PI) rotate @4C for 5min centrifuge @8000rpm for 1min, remove supernatant. 27. Wash with TSE III once (+DTT, PI) rotate @4C for 5min centrifuge @8000rpm for 1min, remove supernatant. 28. Wash with TE twice rotate @4C for 5min centrifuge @8000rpm for 1min, remove supernatant. 29. Remove all supernatant and add 400ul of elution buffer (0.1M NaHCO 3, 1%SDS) Don’t forget Total. Add elution buffer to total tube to make 400ul. Rotate @RT for 20min ~1hour <Reverse Cross-linking> 30. After elution, spin @8000rpm for 1min. Transfer only supernatant to new tubes 31. Add NaCl to every tube. Final NaCl concentration is 200mM. Add 16ul of 5M NaCl to tube. Incubate @65C for at least 6hrs. <Purification of DNA>. 32. Add 8ul of 0.5M EDTA, 16ul of 1M TrisHCl pH 6.5 to every tube. At the same time, add 1.5ul of 25mM Proteinase K to every tube. Incubate @65C for additional 30min~1hour 33. Chloroform extraction Add 400ul of CHCl3 to every tube Vortex very well and spin @13000rpm for 5min 34. Meanwhile, label new tubes and add 1ul of yeast tRNA, 35ul of NaOAc(pH7.0) to each tube. 35. Transfer upper layer about 350ul to new tubes and add 870ul of 100% EtOH. Vortex very well and store @-20C for at least >2hrs. <PCR> 36. Spin @13000rpm, 4C for 30min Remove supernatant and air-dry for 10min. 37. Dissolve DNA pellet with 50ul of TE buffer or DNase free water For total, add 100ul of TE or water. Vortex very well to resuspend pellet completely. 38. PCR step DNA sample : 5ul (total : 2ul ) 10X PCR bf : 5ul 50mM MgCl2 : 3.5ul 25mM dNTP : 0.35ul 100uM Primer F : 0.1ul 100uM Primer B : 0.1ul Taq polymerase : 0.2ul PCR H2O : 35.7ul 50ul /rxn