CONVERSION FACTORS AND
advertisement

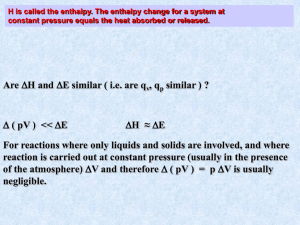
CHEMISTRY 51, FALL, 2004 SOLUTION KEY TO SECOND MIDTERM EXAM CONVERSION FACTORS AND DATA REQUIRED TO ANSWER THE QUESTIONS 1) constants and conversion factors R = 8.31451 J/K-mole = 0.0820578 L-atm/mole-K 1 L-atm = 101.325 J NA = 6.022 x 1023 molecule/mole F = 96485 C/mole 0C = 273.15 K RTln(10)/F = 0.05916 V at 25C 2) thermodynamic data at 25.00C (used in Problem 1) species Cl-(aq) Pt(NH3)3Cl+(aq) Hf(kJ/mole) -167.159 -396.2 S(J/K-mole) 56.5 113 PtCl4-2(aq) -499.2 155 NH3(aq) -80.29 111.3 3) thermodynamic data at 25.00C for the process M+(g) M+(aq) (used in Problem 2) atomic number 3 11 19 37 55 species, M+ Li+ Na+ K+ Rb+ Cs+ H(kJ/mole) -964.273 -849.48 -766.64 -741.27 -716.24 S(J/K-mole) -199.5 -88.8 -52.0 -42.7 -36.67 4) selected standard reduction potentials at 25.00C (used in Problem 3) half reaction E(V) + Ag (aq) + e Ag(s) 0.800 +3 Fe (aq) + 3 e Fe(s) -0.037 Ag(CN)2-(aq) + e- Ag(s) + 2 CN-(aq) -0.407 -3 Fe(CN)6 (aq) + 3 e Fe(s) + 6 CN (aq) -0.527 5) data required for Problem 4. a) enthalpy of combustion of gaseous octane to form liquid water, -5511.8 kJ/mole [H = -5511.8 kJ/mole for C8H18(g) + 12.5 O2(g) 8 CO2(g) + 9 H2O(l)] b) enthalpy of vaporization of water, 44.012 kJ/mole [H 44.012 kJ/mole for H2O(l) H2O(g)] NAME____________________________________ Chem 51, 2004, 2nd. midterm exam The insert contains the values of fundamental constants and data used in the following problems. You may use the back for scratch work but enter ALL work to be graded in the space provided with each question. Show your work in all questions involving computation to receive any credit. The exam has 4 questions on 3 pages. 1) (47 points) Ammonia complexes of platinum(II) are used in cancer chemotherapy. These complexes are often prepared from a chloro complex. Using data provided on the inset, calculate at 35.00C the value of the equilibrium constant for the reaction PtCl4-2(aq) + 3 NH3(aq) 3 Cl-(aq) + Pt(NH3)3Cl+(aq) (Hint: As an intermediate step in the problem, calculate the values of H and S.) Data are given at 298 K. We shall assume that the enthalpy and entropy changes calculated at 298 K are independent of T. H(308.15 K) H(298.15 K) = 3(-167.159) + 1(-396.2) -(-499.2) -3(-80.29) = -157.607 kJ = -157607 J S(308.15 K) S(298.15 K) = 3(56.5) + 1(113) - 3(155) - 3(111.3) = -206.4 J/K G(308.15 K) = H(308.15 K) - (308.15 K)S(308.15 K) H(298.15 K) - (308.15 K)S(298.15 K) = (-157607 J) - (308.15 K)(-206.4 J/K) = -157607 + 63202 = -94005 J K = exp(-G/RT) = exp[-(-94005)/((308.15)(8.31451))] = exp(36.69) = 8.6 x 1015 2) (30 points) You will find on the inset thermodynamic data at 25.00C for the hydration of alkali metal cations, i.e. the elementary reaction M+(g) M+(aq). Write a succinct essay on this process as informed by the thermodynamic data. The process is exothermic because of energy lowering due to solvation of the ions greatly exceeds the energy costs required to separate water molecules. In magnitude, the change is greatest for the smallest ion, Li+. A small radius results in a larger electric field and greater attraction for the water molecules. The water molecules are organized by the solvation process so the entropy change is negative. In magnitude, the greatest change occurs with the smallest ion. Finally, in comparing -TS and H, we note that the overall process is dominated by enthalpy. 3) (25 points) Sleazy Sam plans to pay for his new Porsche by extracting residual silver from old mine dumps. He extracts the silver using a 0.1 M sodium cyanide solution and converts the resulting silver complex to silver metal electrochemically with an iron anode. In his cell, the concentrations of Ag(CN)2- and Fe(CN)6-3 are 1.0 x 10-6 M and 0.10 M, respectively. Calculate the reversible potential of the cell. cell reaction: 3 Ag(CN)2-(aq) + Fe(s) 3 Ag(s) + Fe(CN)6-3(aq) The two half reactions are Ag(CN)2-1aq) + e- Ag(s) + 2 CN-(aq) E(R) = -0.407 V Fe(s) + 6 CN-(aq) Fe(CN)6-3(aq) + 3 e- E(O) = -(-0.527 V) = 0.527 V E = E(R) + E(O) = (-0.407 V) + (0.527 V) = 0.120 V E = E - (0.05916/n)log10(Q), Q = [Fe(CN)6-3]/[ Ag(CN)2-1]3 = 0.120 V - [(0.05916 V)/3]log10[(0.1)/(1.0 x 10-6)3] = 0.120 V - (0.05916/3)(17) = 0.120 - 0.335 = -0.215 V 4) (48 points) This problem will illustrate the application of thermodynamics to the functioning of an internal combustion engine. Suppose that one drop of octane (0.00050 mole) and an excess of air are introduced into a piston. When the spark plug fires and initiates combustion, the volume of the piston is 0.40 L. a) In the piston, the octane is in the gas phase and gaseous water is produced. The combustion reaction is C8H18(g) + 12.5 O2(g) 8 CO2(g) + 9 H2O(g). Using the data on the inset, calculate the enthalpy change for this reaction with the 0.00050 mole of octane. (Hint: use a simple thermodynamic cycle.) We obtain the net reaction by adding two reactions: net = A + 6(B); hence, H(net) = H(A) + 6(B). A: C8H18(g) + 12.5 O2(g) 8 CO2(g) + 9 H2O(l), H(A) = -5511.8 kJ B: H2O(l) H2O(g)], H(B) = 44.012 kJ H(net) = -5511.8 + 6(44.012) = -5511.8 +396.1 = -5115.69 kJ/mole of octane H(rxn) = noctaneH(net) = (0.00050)(-5115.69 x 1000) = -2558 J b) Calculate the energy change for the combustion. Assume a temperature of 300.0 K. We use the definition of H, H = E + pV and the result that pV per mole is negligible for condensed phases and RT for gases. Therefore, H = E + pV = E + RTngas. For the above reaction, pV = (0.0005 mole)[9RT + 8RT -12.5RT -RT] =(0.0005)(-3.5)(RT) = -4.3 J so -2558 J = E + 4.3 J so E = -2554 J. c) The piston expands from 0.40 L to 2.00 L against a constant external pressure. Assume that the internal combustion engine converts 33% of the energy into useful work. Calculate the value of this pressure in atm. [If you are unable to solve part (b), use E = -3000 J for parts (c) and (d).] w = 0.33E = (0.33)(-2554 J) = -851 = (-851 J)/(101.325 J/L-atm) = -8.40 L-atm.. w = -pextV(piston) so pext = -w/ = -(-8.40 L-atm)/(2.0 - 0.4 L) = 5.25 = 5.3 atm. d) The balance of the energy change ends up as waste heat. Assume that the radiator is a water bath with 1.00 x 104 g. The specific heat of water is 4.184 J/gK. Calculate the change in temperature of the water in the radiator. qσ = 0.67E = (0.67)(-2554) = -1711 J qө = -qσ = 1711 J = CөTө and Cө = (4.184 J/K-g)(10,000 g) = 41840 J/K. Tө = qө/Cө = (1711 J)/(41840 J/K) = 0.041 K.