Exempt vs. Non-Exempt application information
advertisement

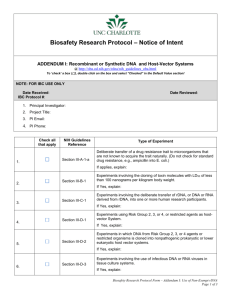
2657/113 Recombinant DNA……To Exempt or Non-Exempt…….That is the Question? By Debra Howeth, MPH What is recombinant DNA (rDNA) you may be asking yourself? The National Institutes of Health Office of Biotechnology Activities (NIH-OBA) defines rDNA molecules as either: (i) molecules that are constructed outside living cells by joining natural or synthetic DNA segments to DNA molecules that can replicate in a living cell, or (ii) molecules that result from the replication of those described in (i) above. In other words, it is genetically engineered DNA prepared by transplanting or splicing genes from one species into the cells of a host organism of a different species. Such DNA becomes part of the host's genetic makeup and is replicated. The Recombinant DNA Program of the NIH-OBA promotes scientific advancement and safety in the conduct of basic and clinical rDNA research. The NIH Guidelines for Research Involving Recombinant DNA Molecules (Guidelines) provides standards guiding containment and safe research practices for rDNA. As a condition for NIH funding of recombinant DNA research, institutions shall ensure that such research conducted at or sponsored by the institution, irrespective of the source of funding, shall comply with the NIH Guidelines. This means that even if you are not receiving NIH funding you are still required to conform to the NIH Guidelines for rDNA. The penalty of non-compliance by any researcher at the institution will result in: forfeiture of funding; suspension or other limitation of financial assistance; and a requirement for prior NIH approval for any or all rDNA projects at the institution. The Guidelines require that institutions ensure that the research is conducted in full conformity with the provisions of the NIH Guidelines. This is the responsibility of the Institutional Biosafety Committee (IBC). At the University of South Florida, the IBC requires registration for the use, possession, storage, and/ or transport of infectious agent(s) (e.g. bacteria, viruses, parasites, fungi, protozoa, prions, etc.), biological toxin(s), recombinant DNA (rDNA) and/or Select Agent(s)/Toxin(s) regulated by the CDC and/or the USDA. So you may be thinking……now what?? How do I know where my research falls under these Guidelines? The first thing that you might do is cruise over to the NIH-OBA website to review the Guidelines for rDNA. Once you have perused the Guidelines, you will realize that the Guidelines are long and very complicated and there are some questions to ask yourself before you can determine where your research falls in terms of the Guidelines. The scope of the rDNA work will determine what type of registration for your rDNA work is necessary. Here at USF, IBC registration of rDNA falls into two categories: Exempt (Requires IBC Notice Simultaneous with Initiation) and Non-exempt (Requires IBC Approval before Initiation). The Exempt applications are reviewed through an expedited process within 5 business days and can be submitted at anytime. Non-exempt applications require review by the full IBC at their monthly meeting. Deadlines for submission and the IBC meeting schedule can be found at http://www3.research.usf.edu/dric/biosafety/meeting-schedule.asp. So how do you know which category your research falls into? Does your research involve…. A construct contain viral DNA that represents more than 2/3 of any eukaryotic viral genome Using risk group 2, 3, or 4 agents as host or vector Cloning DNA for risk group 2, 3, or 4 agents into nonpathogenic prokaryotic or lower eukaryotic host-vector systems Using infectious or defective DNA or RNA viruses in the presence of helper virus in tissue culture systems. Creation of transgenic animals or plants Using more than 10 liters of culture. Cloning of toxin molecules with LD50 of less than 100 ng per kg of body weight. Deliberate transfer of recombinant DNA, or DNA or RNA derived from rDNA into one or more human subjects. Deliberate transfer of a drug resistant trait to microorganisms not known to acquire this trait naturally, if such acquisition could compromise the use of the drug to control disease agents in humans, veterinary medicine, or agriculture. If you can answer “No” to all of these questions, then your rDNA research falls into the exempt category. Some examples of exempt rDNA work would include: 1) The use of plasmids to transfect a cell line to express a gene of interest such as luciferase for imaging. 2) The insertion of rDNA into an expression host such as E. coli K12. On the other hand a “Yes” answer to any of these questions would require the submission of a non-exempt rDNA application. Some types of experiments that would fall into this category would be: 1) Creation of transgenic mice by inserting rDNA into the germ line of the mouse. 2) A viral vector (including viral vector kits) used to transport rDNA into an animal or cells. 3) rDNA used in a human subject such as a viral vector vaccine to deliver a gene of interest. For more information contact the USF Biosafety Program: All IBC applications/forms can be found on our website at: http://www3.research.usf.edu/dric/biosafety/forms.asp Debra Howeth (dhoweth@usf.edu) 813-974-5091 Farah Moulvi (fmoulvi@usf.edu) 813-974-0954 http://www3.research.usf.edu/dric/biosafety/