rDNA EXEMPT FORM - Texas A&M University
advertisement

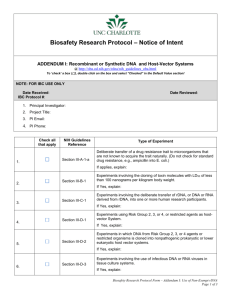
PI Name: Texas A&M University-Kingsville Institutional Biosafety Committee Office of Research and Sponsored Programs: (361) 593-3344 or osr@tamuk.edu rDNA EXEMPT FORM (Once acknowledged by the IBC as Exempt, this study will remain Exempt unless there are substantial changes to this study or the NIH Guidelines) 1. Does the construct contain viral DNA that represents more than 1/2 of any eukaryotic viral genome or is the viral construct from DNA of Risk Group 2, 3, 4* viruses or restricted agents? Yes No Use the links below to determine relevant Risk Group: http://oba.od.nih.gov/rdna/nih_guidelines_oba.html http://www.cdc.gov/od/sap/docs/salist.pdf 2. Does the study involve the deliberate transfer of rDNA; or DNA or RNA that is derived from rDNA into humans, other vertebrates, invertebrates, or plants; or consist of DNA transferred from a prokaryotic or eukaryotic host into a host that is not a closely related strain or species? Yes No 3. Does this study involve the use of a microorganism from a Risk Group 2, 3, 4* or select agent as a Host-Vector System, or cloned DNA from a Risk Group 2, 3, 4 into nonpathogenic prokaryotic or lower eukaryotic Host-Vector System, or if using RG-2 organisms, does it involve the movement of DNA between organisms from different Appendix A sublists? Use the links below to determine relevant Risk Group, select agent, or natural exchanger sublist of your research materials: http://oba.od.nih.gov/rdna/nih_guidelines_oba.html http://www.cdc.gov/od/sap/docs/salist.pdf 4. Does this study involve the generation of toxins lethal for vertebrates at an LD50 of less than 100 µg/kg of body weight? Yes No 5. Does the research involve the generation of more than 10 liters of culture at one time? Yes No 6. Does this study involve the deliberate transfer of a drug resistance trait to microorganisms that are not known to acquire the trait naturally and if so, could this acquisition compromise the use of the drug to control the disease agents in humans, animals and/or plants? Yes No 7. Does your project require the use of BL21 E.coli strain? Yes Revised: 10/29/13 No 1 PI Name: If you answered No to all of the above questions, the study is EXEMPT. If you answered Yes to any of the above questions this study is NON-EXEMPT. Please complete the Recombinant DNA and Artificial Gene Transfer Form. *Risk group classifications can be found in Appendix B of the NIH Guidelines You have reached the end of this form. Please make sure that you have responded to every question on this application . Save an electronic copy of this completed form and submit to osr@tamuk.edu. You will receive an electronic message from the Research Compliance Liaison within 2-3 days acknowledging receipt of your submission. Submit to: Research Compliance Liaison Email as PDF to: OSR@tamuk.edu Mail Code: MSC 201 Fax: 361-593-3409 Revised: 10/29/13 2