Non-Surgical Procedure Descriptions
advertisement

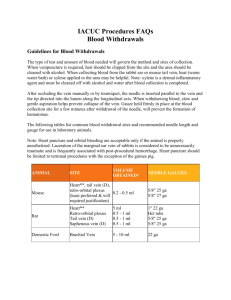
NON-SURGICAL PROCEDURE DESCRIPTIONS BLOOD COLLECTION (AXILLARY CUTDOWN)): This is a terminal procedure with the objective to collect the maximum amount of blood possible. 1. The animal will be anesthetized. 2. With the animal in dorsal recumbency (lying on its back), the axillary (armpit) area will be prepped with alcohol swab. 3. A cut will be made in the axillary region with scissors or a scalpel blade to expose the subclavian artery and vein which are deep in the armpit. 4. The subclavian artery and vein will be cut with the scissors or a scalpel blade. 5. The blood sample will be collected with the syringe (no needle) as the blood pools in the axillary region. 6. After the procedure, a thoracotomy will be performed to assure the animal has been euthanized. BLOOD COLLECTION (CARDIAC): This is a terminal procedure with the objective to collect the maximum amount of blood possible. 1. The animal will be anesthetized. 2. With the animal in dorsal recumbency (lying on its back), the area will be prepped with an alcohol swab. 3. A needle will be inserted at base of sternum at a 20-30 degree angle just lateral of the midline on the animal's left side. The thumb and index finger will be used to feel the heartbeat to assist in directing the needle. 4. The syringe will be aspirated slowly while advancing. 5. Alternatively, the procedure may be performed with the animal on its right side. In this case the needle will be inserted just behind the forelimb where the heartbeat is easily felt. 6. After the procedure, a thoracotomy will be performed to assure the animal has been euthanized. BLOOD COLLECTION (FEMORAL VEIN): 1. The animal will be anesthetized. 2. Animal will be placed in dorsal recumbency (lying on back) 3. Pulse of femoral vein will be palpated and the skin will be swabbed with alcohol. 4. A needle will be inserted into vein for blood collection using a vacutainer collection system. 5. Upon completion, gentle pressure will be applied to the collection site to insure hemostasis. 6. Regarding collection amount, no more than 1% of the animal's body weight will be collected in a two week interval. For example, if the animal weight 100 grams, no more than 1 gram of blood (equivalent to 1 ml of blood) will be collected in a two week interval. 7. Regarding collection frequency, XXXX BLOOD COLLECTION (RETRO-ORBITAL): 1. The animal will be anesthetized. 2. After the animal is anesthetized, a drop of Proparacaine Hydrochloride (local anesthetic) will be placed in the eye from which the sample is to be collected. The Proparacaine Hydrochloride takes effect in about 30 seconds and lasts for about 15 minutes. 3. A hematocrit tube will be placed at the medial canthus of the eye and inserted behind the eye. 4. The tube will be rotated gently against the back of orbit until blood flows. Please note that this is a finesse procedure and does not require force. 5. Sterile eye ointment will be instilled when finished. 6. Upon completion, good hemostasis will be insured by holding eyelids closed. 7. Regarding collection amount, no more than 1% of the animal's body weight will be collected in a two week interval. For example, if the animal weight 100 grams, no more than 1 gram of blood (equivalent to 1 ml of blood) will be collected in a two week interval. 8. Regarding collection frequency, XXXX BLOOD COLLECTION (SAPHENOUS): 1. The animal will be restrained. 2. Hair will be clipped from lateral aspect of lower leg. A straight razor may also be used to clip the fur from the leg. 3. The saphenous vein will be lightly constricted above the knee joint and petroleum jelly applied over the shaved area of the leg as this will facilitate blood flow and help prevent clotting during collection. 4. The vein will be punctured with a needle (typically 23 gauge). Blood will be collected with a hematocrit tube or other collection tube. 5. Upon completion, good hemostasis will be insured by applying gentle pressure to the collection site. 6. Regarding collection amount, no more than 1% of the animal's body weight will be collected in a two week interval. For example, if the animal weight 100 grams, no more than 1 gram of blood (equivalent to 1 ml of blood) will be collected in a two week interval. 7. Regarding collection frequency, XXXX BLOOD COLLECTION (TAIL NICK): 1. The animal will be restrained. 2. Tail will be cleaned with alcohol. 3. The tail veins are located on either side of the tail. Starting midway up the tail, the vein will be nicked with a sterile scalpel blade. Blood may be collected with micro capillary tubes, a micropipette or various microtainer collection tubes. 4. Upon completion, good hemostasis will be insured by applying gentle pressure to the collection site. 5. Subsequent blood samplings may be obtained in animals by removing the scab 6. Regarding collection amount, no more than 1% of the animal's body weight will be collected in a two week interval. For example, if the animal weight 100 grams, 7. no more than 1 gram of blood (equivalent to 1 ml of blood) will be collected in a two week interval. Regarding collection frequency, XXXX BLOOD COLLECTION (TAIL SNIP): 1. The animal will be restrained. 2. Tail will be cleaned with alcohol. 3. With a sterile scalpel blade, a transverse section will be made through the long axis of the tail 2 mm from the tip. 4. A hematocrit tube or other blood-collecting tube will be used to collect blood dripping from the sectioned tail. 5. The tail will be massaged by passing the thumb and index finger from the base to the tip of the tail if blood flow is inadequate. 6. Upon completion, good hemostasis will be insured by applying gentle pressure to the collection site for 1 minute. Blood cauterization (e.g. via application of a silver nitrate stick) may be used as indicated. 7. Repeated blood sampling may be obtained by two methods with method used based upon length of time after the initial incision. 0 - 24 hrs.: Bleeding may be restarted by removing the clot; always try this method first...!!! > 24 hrs.: Follow the above protocol; however, make the new cut through the tail 1 mm from tip. Only the fleshy portion of the tail tip should be cut. No cutting into vertebrae is permitted. As only a small portion of the tail does not contain vertebrae, the use of the tail cut procedure should be limited. Accordingly, no more than one snip in addition to the original snip is permissible unless otherwise specified and approved by the IACUC. 8. Regarding collection amount, no more than 1% of the animal's body weight will be collected in a two week interval. For example, if the animal weight 100 grams, no more than 1 gram of blood (equivalent to 1 ml of blood) will be collected in a two week interval. 9. Regarding collection frequency, XXXX BLOOD COLLECTION (WING VEIN): 1. Birds will be captures by hand from one of the large flight aviaries (this is done by switching off the lights and inducing perching behavior then gently capturing the bird with both hands). 2. The birds will be gently restrained with one hand while the other hand fully extends one wing. 3. A second person will then locate the alar vein on the inside of the wing (there are few, if any, feathers here and plucking them is not necessary) and washes it with 70% alcohol. 4. The alar vein will be gently punctured with a sterile hypodermic needle. The blood will be collected into 2 or 3 heparinized capillary tubes (150-210 microliters total). 5. To stop excess bleeding gelfoam powder can be applied, but often gentle pressure with a gauze pad for 30 seconds is more than necessary to stop the bleeding. 6. 7. Regarding collection amount, no more than 1% of the animal's body weight will be collected in a two week interval. For example, if the animal weight 100 grams, no more than 1 gram of blood (equivalent to 1 ml of blood) will be collected in a two week interval. Regarding collection frequency, XXXX EYE INJECTION: 1. The animal will be anesthetized. 2. The goal is to trace the extent of retinal projections. It will be done under modified sterile conditions because the surface of the eye is not sterile and cannot be given a sterile scrub. Atropine (0.4 mg/kg SQ) and Doxapram (2 mg/kg SQ) will be given to counteract bradycardia and apnea and to reduce mucosal secretions. 3. After anesthesia, the pupils will be dilated with atropine and phenylephrine eyedrops to improve visualization of the injection needle. Proparacaine ophthalmic drops will be placed in the eye for additional pain relief (topical corneal anesthesia). 4. The eyeball will be rotated gently by pulling on the conjunctiva with a forceps, and a 25 gauge Hamilton syringe will be inserted into the orbit caudal to the anterior chamber and lens. The syringe contains 1-10 n1 (depending on age) of an anterograde tracer (2% wheat germ-agglutinated horse radish peroxidase (HRP) or 10% cholera toxin subunit B, both of which are non-toxic) and this will slowly be injected. 4. The needle will then be withdrawn and a topical ophthalmic antibiotic (BNP) will be administered. After the animal recovers from anesthesia, in addition to the topical BNP antibiotic ointment, analgesics (Buprenorphine, 0.01 mg/kg SQ), and an anti-inflammatory (Dexamethasone, 1mg/kg IM) will be given as needed (if redness or swelling appears) throughout the survival period. INTRAVENOUS DRUG SELF-ADMINISTRATION: Subjects will be tested in a standard operant chamber with retractable levers that trigger syringe pumps to administer drug solution through polyethylene tubing connected to the i.v. catheter. Most self-administration sessions will be 3 hr or shorter, in which case no food or water will be available. In longer sessions, food and water will be present in the operant chamber. Drugs used in self-administration paradigms will include XXXX (SPECIFY INFUSION RATES AND DOSAGES HERE). Frequency of infusion is XXXX (e.g. daily for XXXX days). IN VIVO MUSCLE STRENGTH TESTING: The animal will be anesthetized. The purpose of this procedure is to evaluate the contractile function (i.e., torque-frequency relationship) of the left anterior crural muscles. Using a multiple use experimental protocol (i.e., J. Physiol. 515: 609, 1999; J. Appl. Physiol. 97: 1067-1076, 2004; J. Appl. Physiol. 98: 1674-1681, 2005), we have observed no adverse effect on the health of the mouse (i.e., no loss in body weight, decrease in grooming habits, or in muscle functional capacity of control mice). After anesthesia and aseptic preparation of the left leg, the mouse will be transferred to a temperature-controlled platform. The ankle and knee will be held in place using the same device described in J. Appl. Physiol. 72:1205, 1992 and used previously by the investigator (i.e., J. Appl. Physiol. 79:1260, 1995; J. Appl. Physiol. 80:332, 1996; J. Mus. Res. Cell Motil. 19:215, 1998; J. Appl. Physiol. 84:2171, 1998; J. Appl. Physiol. 85:58, 1998; J. Physiol. 515:609, 1999; Med. Sci. Sport Exerc., 32: 820, 2000; J. Appl. Physiol. 97: 1067-1076, 2004; J. Appl. Physiol. 97: 1619-1625, 2004; J. Appl. Physiol. 98: 16741681, 2005). Two sterilized platinum subdermal needle electrodes will be inserted into the aseptically prepared skin proximal to the common peroneal nerve on the left hindlimb. Anterior crural muscle strength will be measured by stimulating the left common peroneal nerve via percutaneous needle electrodes. Anterior crural muscle torque will then be measured at the following stimulation frequencies: 20, 40, 60, 80, 100, 125, 150, 200, 250, 300, 350, and 400 Hz. PLETHYSMOGRAPH CHAMBER RECORDING: Frequency the procedure will be conducted: XXXX Animals will be exposed to high CO2 (INDICATE VARIOUS CONCENTRATIONS HERE) in the plethysmograph chamber. Respiratory frequency, amplitude and breathing patterns will be recorded using a force-electricity transducer. The exposure period for each concentration will be (XXXX minutes) followed by a 30 minute recovery. The same experiment will be repeated once if necessary. Thus, each individual animal will be evaluated no more than twice in the chamber. The specified concentrations of CO2 do not seem to cause distress in animals as the animals do not show any behavioral abnormalities during the conduct of the procedure. The animal will be placed in the plethysmograph chamber (40 ml volume), which will be connected to another chamber with the same volume to be used as a reference chamber connected to the recording chamber through tubing. The chambers will be constantly ventilated with air with a flow rate of about 50 ml/minute. The chamber will be flushed with a mixture of 6% CO2 balanced with air for 6 minutes during the experimental period, which is the hypercapnia challenge. Breathing activity is barometrically recorded continuously by measuring the pressure changes inside the chamber with the transducer, an amplifier, an A/D converter, and the Axoscope 9 software. Following the procedure, the mouse will be returned to its home cage. SUBSTANCE ADMINISTRATION: The following represent various routes of administration for one to consider. Certainly, others may apply. Intramuscular (IM) Injection * Specify injection volume and frequency: XXXX 1. The animal will be restrained. 2. Area will be cleaned with alcohol. 3. Needle will be inserted into hind leg muscle (either in front of the thigh bone or, if behind it, direct the needle towards the back of the leg). 4. Syringe will be aspirated to insure proper placement. Any sign of blood in the syringe indicates improper placement- reposition. 5. Article will be administered in a steady, fluid motion. DO NOT administer rapidly because this may cause tissue trauma. Subcutaneous (SC) Injection * Specify injection volume and frequency: XXXX 1. The animal will be restrained. 2. Area will be cleaned with alcohol. 3. Needle will be inserted at base of skin fold between thumb and opposing finger. 4. Syringe will be aspirated to insure proper placement. Any sign of blood indicates improper placement; also, a lack of negative pressure in the syringe indicates the needle has punctured out through the opposite side of the skin - reposition. 5. Article will be administered in a steady, fluid motion. Intraperitoneal (IP) Injection * Specify injection volume and frequency: XXXX 1. The animal will be restrained, tilting the body at a 45-degree angle with the head down. This will position the intestines cranial to the injection site. 2. Area will be cleaned with alcohol. 3. A needle will be inserted into the animal's right lower quadrant of the abdomen at a 30-degree angle. 4. Syringe will be aspirated to insure proper placement. Any sign of blood or other fluid indicates improper placement. To prevent inducing peritonitis, remove syringe, discard, and use new syringe, needle, and article in the event that fluids other than blood are aspirated. 5. Article will be administered in a steady, fluid motion. Intradermal (ID) Injection * Specify injection volume and frequency: XXXX 1. The animal will be anesthetized. 2. The hair on the back will be clipped and prepped with an alcohol swab. 3. A needle will be inserted between layers of skin on the back at a 30-degree angle. 4. Syringe will be aspirated to insure proper placement. Any sign of blood or other fluid indicates improper placement- reposition. 5. Article will be administered in a steady, fluid motion to avoid tissue trauma. Successful injection results in a small circular skin welt. Intravenous (IV) Injection Utilizing Lateral Tail Veins Note: The secret of successful injection of the tail vein is to dilate the veins. This has been accomplished in various ways such as the following: placing the tail in warm water (47 degrees Celsius for about 1 minute (do not exceed 47 C as this can result in thermal injury to the tail); placing the animal in an incubator (37° C) for 10 to 15 minutes; carefully using a heat lamp on the tail for 1-2 minutes; or wrapping the tail in an electric heating pad that is warm (not hot) to the touch. In addition one can place a tourniquet around the base of the tail to facilitate visualization of the vein (a rubber band and mosquito hemostat are suitable for this purpose). * Maximum injection volume = ~1% of the animal's body weight in mls (i.e., 0.3 mls for a 30 g mouse) 1. The animal will be restrained. 2. The tail will be warmed as mentioned above to facilitate dilation. 3. Needle placement will be no closer to the body than half the length of the tail. 4. With tail under tension, the needle will be inserted into skin approximately parallel with the vein. 5. Proper placement will be insured by inserting needle at least 3 mm into lumen of vein. 6. Article will be administered in a steady, fluid motion to avoid rupture of vessel. 7. Upon completion, good hemostasis will be insured by applying gentle pressure before returning to cage. Gavage * Maximum administration volume = 5ml/kg (this equals 0.15 ml for typical adult mice) 1. The distance from the tip of nose to the last rib will be measured as this constitutes the length gavage tube will be inserted be inserted. 2. The animal will be restrained 4. The tip of gavage tube will be placed in the rear of the animal’s mouth to induce swallowing. 5. The tip will be slid down back of mouth, moving tip forward in one fluid motion. 6. Take your time, any resistance felt indicates improper placement. Tube should slide down into esophagus easily. A violent reaction (coughing, gasping) usually follows accidental introduction of the tube into the larynx or trachea. 7. Using the gavage tube to gently extend the neck facilitates introduction into the stomach. 8. Once the needle is properly placed, the article will be administered in a steady, fluid motion. VAGINAL LAVAGE: Frequency the procedure is conducted: XXXX Vaginal smears will be obtained by saline infusion into the vagina. Animals will be grasped by the base of the tail, drawn to the front of the cage or bench, and held against the wall of the cage or top of the bench by applying gentle pressure on their backs. The tail will be lifted and a 1 ml sterile pipette containing a small amount (approximately 200 microliters) of sterile saline will be inserted into the vaginal opening. The saline will be flushed into the vagina, and a sample will be withdrawn and placed onto a clean microscope slide for examination.