Marshmallow Molecule lab
advertisement
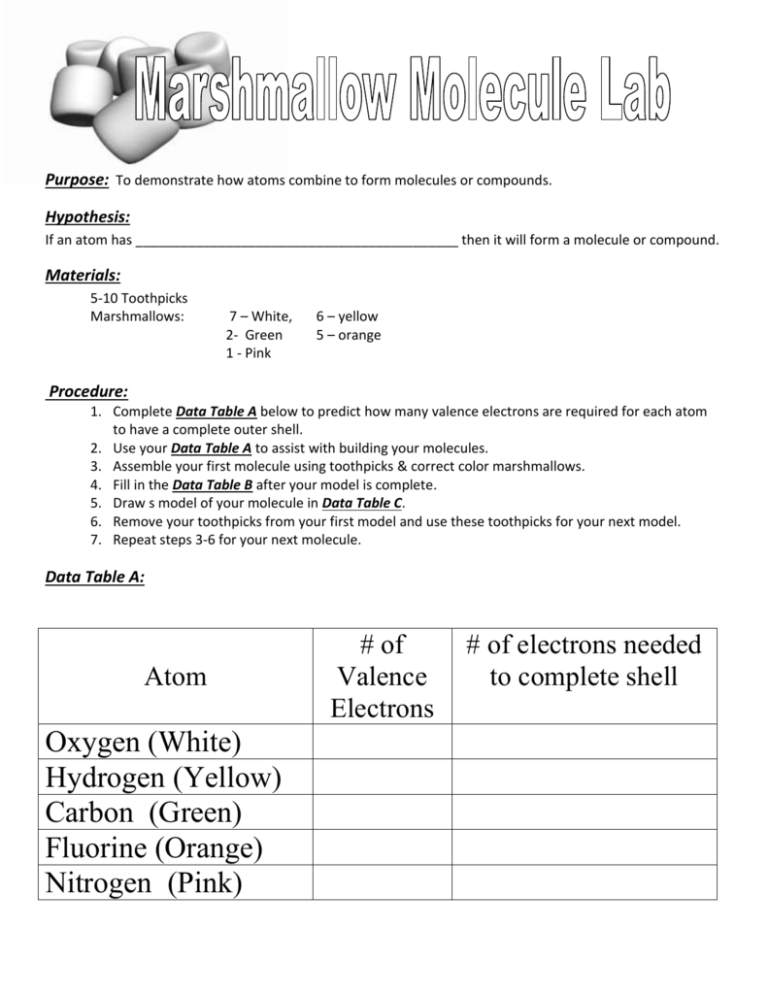
Purpose: To demonstrate how atoms combine to form molecules or compounds. Hypothesis: If an atom has ___________________________________________ then it will form a molecule or compound. Materials: 5-10 Toothpicks Marshmallows: 7 – White, 2- Green 1 - Pink 6 – yellow 5 – orange Procedure: 1. Complete Data Table A below to predict how many valence electrons are required for each atom to have a complete outer shell. 2. Use your Data Table A to assist with building your molecules. 3. Assemble your first molecule using toothpicks & correct color marshmallows. 4. Fill in the Data Table B after your model is complete. 5. Draw s model of your molecule in Data Table C. 6. Remove your toothpicks from your first model and use these toothpicks for your next model. 7. Repeat steps 3-6 for your next molecule. Data Table A: Atom Oxygen (White) Hydrogen (Yellow) Carbon (Green) Fluorine (Orange) Nitrogen (Pink) # of Valence Electrons # of electrons needed to complete shell Data Table B: Chemical Formula Oxygen O2 Water H2O Carbon Dioxide CO2 Carbon Tetrafluoride CF4 Nitrogen Trihydride NH3 Number of atoms of Elements in the Molecules Oxygen Hydrogen Carbon Fluorine Lithium (White) (yellow) (green) (Orange) (Pink) Total # of Atoms Data Table C: O2 H2O CO2 CF4 NH3 Analysis questions: Answer these questions when you lab is complete. 1. How many valence electrons do these atoms have in its outer shell? How many more do they need to become stable? Sodium __________ , __________ Nitrogen __________ , __________ Neon __________ , __________ 2. Why do atoms combine? 3. Build and draw two water molecules. Can you use these 2 water molecules to create 2 separate O2 and two H2 molecules? 4. Did you have to change any of the chemical bonds (toothpicks) in the above water molecules to form the individual H and O molecules? 5. Do you think the H and O molecules have the same or different physical/chemical properties than the water molecules?