Phytoplankton sampling and community description
advertisement

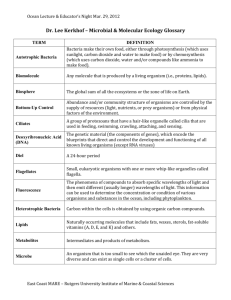
Online supporting Information Appendix S1 Phytoplankton sampling and community description Phytoplankton samples processing Quantitative samples were fixed with Lugol’s solution and counted under an inverted microscope at 400x magnification using the settling technique of Utermöhl (1958). Picoplankton (organisms smaller than 2 µm in diameter) cannot be identified using this technique and thus were not considered. The organism was considered as the unit (unicell, colony or filament). Individual volume (V, m3) and surface area (S, m2) were calculated according to Hillebrand et al. (1999), based on geometric shapes and average dimensions (length, width and depth, in µm) of 5–40 organisms per taxa, measured in each sample under a transmitted light microscope at 1000x magnification. Also recorded were cell numbers per colony and organism dimensions, including the maximum linear dimension (MLD, m). The presence of categorical traits, including aerotopes, flagella, mucilage, heterocytes and siliceous exoskeletal structures, were noted for each relevant organism. Density of biomass was expressed as biovolume (m3 L-1) by multiplying sample abundance (org L-1) by species individual volume (m3). Duplicate samples of 350 ml fixed with Lugol solution were used for quantitative analyses. Samples were gently homogenized and poured into sedimentation chambers of 1 to 10 ml, depending on the density of organisms per sample. Sedimentation time was 4 to 48 hours according to the height of the chamber (Sournia 1978) to allow the settling of big and small organisms. Duplicate phytoplankton counting were performed by transects until obtaining 400 units (cells or colonies) according to Utermöhl (1958), using a Nikon Diaphot inverted microscope (400 X or 1000X) with phase contrast. With this approach it was possible to count, without introducing bias, organisms bigger than 3 µm diameter. The software ALGAMICA 4.0 (Gosselain & Hamilton 2000) was used for counting and calculating abundance per milliliter. Taxonomic identification was based on fresh, fixed and oxidized material (for diatoms). Flagellates less than 10 µm diameter were not possible to identify at specific level, and thus, they were classified in different taxa according to their morphology (size, shape, type of plast, number of flagella). In few cases, diatom species were confirmed under scanning electronic microscope (JEOL JSM-5200). The classification system of Hoeck et al. (1993) was followed for Classes and Divisions, and Krammer & Lange-Bertalot (1986; 1988; 1991), Tomas (1998) and Round et al. (1992) were used as main taxonomic references for genera and species. Morphology-based functional groups (MBFGs) Species were classified into MBFG according to Kruk et al. (2010), based on their individual Volume (V), Surface (S), S/V, Maximum linear dimension (MLD) and the presence or otherwise of five categorical traits named: aerotopes, flagella, mucilage, heterocysts and siliceous exoskeletal structures. This classification included the following seven groups: (I) small organisms with high S/V, (II) small flagellated organisms with siliceous exoskeletal structures, (III) large filaments with aerotopes, (IV) organisms of medium size lacking specialised traits, (V) flagellates unicells with medium to large size, (VI) non-flagellated organisms with siliceous exoskeletons and (VII) large mucilaginous colonies. A detailed description of the MBFGs can be found elsewhere (Kruk et al. 2010). Significance of empirical entropy peaks We determined the significance of entropy (S) peaks by comparing observed entropy against an expected uniform distribution under the null hypothesis of homogeneous entropy. One thousand comunities were created by sampling the volumes of species from a random uniform distribution bounded by observed individual volumes. Then, each species had a biovolume assigned to it, which was taken from a randomization of the observed biovolume matrix, keeping both the empirical species rank-abundance pattern and total biovolume in the sample. Subsequently, entropy was estimated for each segment of the niche as described in methods. For each segment, the observed S value was compared with the distributions of S generated under the null hyphothesis, with significance defined according to standard 5% criterion. Phytoplankton species Representative phytoplankton species of morphology-based functional groups (MBFGs) found in Rocha Lagoon at medium and large size-classes. A detailed list of species can be found in Bonilla et al. (2005). Species representatives Medium (~1000 m3) Large (<10000 m3) MBFG V Eutreptiella gymnastica, NA Trachelomonas sp., Prorocentrum minimum, Pyramimonas sp. and Cryptomonas sp. MBFG VI Nitzschia spp., Fragilaria Melosira moniliformis, sp. and Cyclotella Thalassiosira eccentrica, meneghiniana Surirella splendida, Cylindrotheca gracilis and Nitzschia obtusa REFERENCES Bonilla S., Conde D., Aubriot L. & Pérez M.C. (2005) Influence of Hydrology on Phytoplankton Species Composition and Life Strategies in a Subtropical Coastal Lagoon Periodically Connected with the Atlantic Ocean. Estuaries, 28, 884–895 Gosselain V. & Hamilton P.B. (2000) Algamica: revisions to a key-based computerized counting program for free-living, attached, and benthic algae Hydrobiologia, 438, 139-142 Hillebrand H., Dürselen C., Kirschtel D., Zohary T. & Pollingher U. (1999) Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology, 35, 403424 Hoeck v.D., Mann D.G. & Jahns H.M. (1993) Algae: an Introduction to Phycology Cambridge Universtiy Press, Cambridge. Krammer K. & Lange-Bertalot H. (1986) Bacillariophyceae 1. Teil: Naviculaceae. G. Fisher, Stuttgart,, Jena. Krammer K. & Lange-Bertalot H. (1988) Bacillariophyceae 1. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. G. Fisher, Stuttgart, Jena. Krammer K. & Lange-Bertalot H. (1991) Bacillariophyceae 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. G. Fischer Verlag, Stuttgart, Jena. Kruk C., Huszar V.L.M., Peeters E.T.H.M., Bonilla S., Costa L., Lürling M., Reynolds C.S. & Scheffer M. (2010) A morphological classification capturing functional variation in phytoplankton. Freshwater Biology, 55, 614-627 Round F.E., Crawford R.M. & Mann D.G. (1992) The Diatoms. Biology and morphology of the genera. 2 edn. Cambridge University Press, Cambridge. Sournia A. (1978) Phytoplankton Manual. UNESCO, Paris. Tomas C. (1998) Identifying marine phytoplankton. Academic Press, San Diego. Utermöhl H. (1958) Zur Vervollkomnung der quantitativen Phytoplankton-Methodik. Mitteilungen. Internationale Vereiningung fuer Theoretische und Angewandte Limnologie 9, 1-38