published data1
advertisement

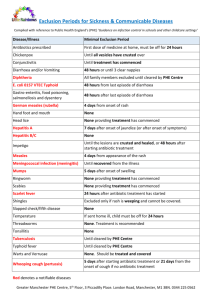
Development and Validation of Spectrophotometric Method for Simultaneous estimation of Levocetrizine Dihydrochloride and Phenylephrine Hydrochloride in their Tablet Dosage Form Urwashi Tiwari 1*, S. P. Wate1, R. S. Jumle Sharad Pawar College of Pharmacy, Wanadongari, Nagpur Corresponding author : tiwariurwashi@yahoo.in ABSTRACT: Simple and sensitive spectrophotometric method has been developed for the estimation of Levocetirizine dihydrochloride (LEC) and Phenylephrine hydrochloride (PHE) in their tablet dosage forms. Method involved solving simultaneous equations based on measurement of absorbances at two wavelengths 230.5 nm and 275.5 nm, theλ max of Levocetrizine dihydrochloride and Phenylephrine hydrochloride, respectively. Beer’s law was obeyed in the concentration range of 2.5 -12.5µg/ml for LEC and 5-25 µg/ml for PHE with line of regression 0.0673x + 0.2386 with correlation coefficient of 0.9983 and 0.3459x – 2.522and correlation coefficient 0.9830 respectively..The mobile selected was based upon the absorption and λmax. Methanol:distilled water (90:10) was used as a solvent for PHE and Levo. Overlain spectra of PHE and LEVO suggested that both the drugs show same absorbance at 250.5 nm i.e. at isobestic point .Commercially available tablets were analyzed and the results are statistically compared with those obtained by the reference method and validated by recovery studies. The results are found satisfactory and reproducible and can be used for routine analysis of both drugs in quality control laboratories. These methods offer the advantages of rapidity, simplicity and sensitivity and normal cost and can be easily applied to resource –poor settings without the need for expensive instrumentation and reagents. KEYWORDS: Levocetirizine dihydrocloride, Phenylephrine hydrochloride, simultaneous equation method, methnol:water(90:10), validation of analytical method. INTRODUCTION: 1,2 Levocetirizine dihydrochloride (LEVO), is R2(2(4(4Chlorophenyl)phenylmethyl)1piperazinyl)ethoxy)acetic acid hydrochloride. It is indiacted for the relief of symptoms associated with seasonal allergic rhinitis (SAR) and perennial allergic rhinitis (PAR), and for the treatment of the uncomplicated skin manifestations of chronic idiopathic urtcaria(CIU).It is official in Indian Pharmacopoeia.3Similarly, Phenylephrine hydrochloride (PHE), is R-2Methylaminol(3hydroxyphenyl)ethanol hydrochloride. It is an alpha –adrenergic receptor, producing pronounced vasoconstriction.4-9 Since no spectrophtometric method had been reported for the simultaneous estimation of Levocetirizine dihydrochloride and Phenylephrine hydrochloride, a successful attempt has been made to estimate both these drugs simultaneously by simple UV spectrophotometric method in their tablet dosage form. Suitable statistical tests were performed on validation data. Few HPLC methods and 10-12 very few UV spectrophotopmetric methods have been reported for simultaneous estimation of Levocetirizine dihyrochloride and Phenylephrine hydrochloride in tablet dosage form in combination with other two drugs in the literature in pharmaceutical formulation. 13 Levocetirizine was officially assayed by Liquid chromatography using stainless steel column 25mm x4.Phenylephrine was also assayed by potntiometrically.Mobile phase was used as 0.05 m potassium dihydrogen phosphate and acetonitrile( 60:40v/v withpH adjusted with and 10% w/v sodium hydroxide, packed with octa desyl silane bonded with silica.14 Phenylephrine hydrochloride was assayed (I.P) by potntiometrically using 0.1 M HCl and 50 ml ethanol (95%) and titrate with 0.1 M ethanolic NaOH. Many researchers have dealt with the development of methods that quantify LEVO in pure and in tablets.15,16 Methods that include validation of UV Spectrophotometric methods for simultaneous estimation of Levocetrizine dihydrochloride in bulk and pharmaceutical formulation when present alone or in combination with Ambroxol hydrochloride. MATERIAS AND METHOD: Levocetirizine dihyddrrochloride and Phenylephrine hydrochloride were obtained from Zim laboratory, Kalmeshwar, Nagpur as a gift sample and were used as working standards. Methanol of analytical grade and double distilled water were used throughout the analysis. The pharmaceutical formulation of LEVO and PHE that is Levocet D +(Heteo Health care,Mumbai) the commercial formulation of Levocetirizine and Phenylephrine are available in ratio of 1:2 as tablet. Instrument: Shimadzu UV/Vis double beam spectrophotometer, model 1700 Pharmaspec with 1 cm quartz cells was used for all spectral measurements. .2HCl Fig-1: Chemical structure of Levocetirizine dihydrochloride .HCl Fig-2:Chemical structure of Phenylephrine hydrochloride Preparation of Standard and sample drug stock solution : Standard stock solution of phenylephrine hydrochloride and levocetrizine dihydrochloride was prepared by dissolving 100mg of PHE and 50 mg of LEVO in 100ml volumetric flask using methanol:water (90:10) as solvent and volume was made up to 100 ml to produce 1000μg/ml of PHE and 500 µg of LEVO. From these stock solutions, working standard solutions of concentration were prepared by appropriate dilutions. Working standard solutions were scanned in the entire UV range to determine the λmax.The λmax of PHE and LEVO were found to be 275.5 nm and 230. 5 nm respectively. Calibration curves: Five standard dilutions of each drug were prepared having concentration of 2.5 – 25 µg/ml, the absorbances of these solutions were measured at 275.5 nm and 230.5 nm and calibration curves were plotted. The absorptiviy coefficients of the two drugs were determined using calibration curves(graph 1and 2). Concentration( µg/ml) Fig-3: Calibration Plot of Phenylephrine Concentration (µg/ml ) Fig-4: Calibration Plot of Levocetirizine Preparation of sample solution: Sample solution containing both the drugs was prepared by dissolving 20 mg of PHE and 10 mg of LEVO in 100ml volumetric flask using 90 ml of methanol and 10ml of double distilled water and made 200 µg/ml and 100µg/ml of PHE and LEVO stock solution respectively.,working standard of 10 µg/ml concentration was prepared by appropriate dilution. Five standard solution of PHE of concentration 5,10,15.20,25 and five standard solution of LEVO of concentration 2.5, 5,7.5,10.5,12.5 were prepared from wokking standard solution.The absorbance of this sample solution was measured at 275.5 nm and 230.5 nm and their concentrations were determined using proposed analytical methods. Simultaneous equation method: Two wavelengths selected for the method are 275.5 nm and 230.5 nm that are absorption maxima of PHE and LEVO respectively in methanol:water (90:10).The absorbasences were measured at the selected wavelengths and absorptivities (A 1%, 1 cm) for both the drugs were determined as the mean of the five independent determinations. Concentrations in the sample were obtained by using following equationsCx = (A2ay1‐A1ay2)/ (ax2ay1‐ax1ay2) …………….1) Cy = (A1ax2‐A2ax1)/ (ax2ay1‐ax1ay2) ……………..2) Where, A1 and A2 are absorbances of mixture at 275.5 nm and 230.5nmrespectively. ax1 and ax2 are absorptivities of PHE at λ1 and λ2 respectively. ay1 and ay2 are absorptivities of LEVO at λ1 and λ2 respectively. Cx and Cy are concentrations of PHE and LEVO respectively. Fig-5: UV Spectrum of PEN Fig-6:UV Spectrum of LEVO Fig-7:UV overlain spectra of PHE and LEVO Estimation in the marketed formulation : Twenty tablets were accurately weighed. Average weight of tablet was calculated. The tablets were crushed to fine powder and mixed thoroughly. A quantity of tablet powder equivalent to 10 mg of PEN and 5 mg of LEVO was transferred to 100 ml volumetric flask and dissolved in distilled water with vigorous shaking and volume was made to 100 ml with same solvent. The solution was filtered through Whatman filter paper no. 41. The aliquot portion of filtrate was further diluted with solvent to get final concentration of about 10 µg/ml and 5 µg/ml of PEN and LEVO respectively. The absorbance of resulting solutions was measured at 230.5 nm and 275.5nm in 1 cm cell against blank.The content of PEN and LEVO in tablet was calculated using the formula as follows. Where, Cx or Cy = Concentration of PEN and LEVO in g/100 ml W = Average weight of tablet Wm = Weight of sample L = Label claim of sample taken Thr result of analysis of the formulation is shown in table 1. Method validation: The method validation parameters Like accuracy, precision,rudgeness, linearity and range were checked as per ICH guidelines.The accuracy of the method was determined by the recovery studies. The recovery studies were performed by the standard addition method at 80%,100% and 120% and the percentage recoveries were calculated and are shown in Table 1 The Precision of the method was evaluated by interday and intraday variation studies.In intraday studies ,working solution of standard and sample were analysed thrice in a day and percentage relatve standard deviation (%RSD) was calaculaed,The data is shown in table2. The linearity for the PHE and LEVO were determined at five concentration levels, ranging from 80-120 % of the test concentration and absorbance were recorded at 230.5 nm and 275.5 nm. PEN and LEVO were found to be linear in 80-120% of the test concentration. Results of linearity and range studies are shown in Table – 2,3. RESULTS AND DISCUSSION : In the present work, new method , namely (Vierordt’s method ) was used for the simultaneous spectroscopic estimation of Phenylephrine HCl and Levocetirizine dihyrochloride in commercially available tablet dosage form. The concentrations in the range of 2.5 – 25 µg/ml of working standard and two sampling wavelengths of 275.5 nm (λ max of PHE HCl), and 230.5 nm (λ max of LEVO dihydrochloride) gave optimum accuracy, precision, economy and sensitivity for this method. The proposed procedure was successfully applied to the determination of PHE and LEVO l in the commercially available tablet dosage form. Recovery studies were carried out at different concentrations by spiking a known concentration of standard drug to the preanalysed sample and contents were reanalyzed by proposed methods.The results of marketed formulation analysis and recovery studies are depicted in table 1.The method was validated statistically for range , linearity, precision,accuracy and results are depicted in table in 2. Accuracy was ascertained on the basis of recovery studies.Precision was calculated as inter ahd intraday variation for boththe drugs. Th recovery experiment indicated the absence of interference from the commonly encountered pharmaceutical excipients present in formulations.The proposed method is found to be simple, sensitive and can be used for routine quality control analysis of PHE and LEVO in bulk and tablet dosage form. Table-1:Result of analysis of recovery study of marketed formulation. Dosage Form Labelled claim % Estimated % Recovery S.D COV(%) S.E Tablet 10 100.70 101.18 0.3159 0.3122 0.4066 5 100.8 100.54 0.2146 0.2134 0.2793 Table-2: Validation parameters for Phenylephrine HCl Parameters Results 1. λmax 275.5 nm 2. Beer’s law limit(ug/ml) 5-25 ug/ml 3. Regression equation a.Slope b.Intercept 0.3459 -2.522 4. Correlation coefficient (r2 ) -2.522 5. Molar absorptivity 35958.61 6. Precision 100.17,99.10 7. Specificity A 10 µg/ml solution of drug in solvent(methanol and distilled water in ratio of 90:10Respectively) at UV detection of 275.5nm will show an absorbance value of 0.1903 Table-3: Validation parameters for Levocetirizine dihydrochloride Parameters Results 1. λmax 230.5 nm 2. Beer’s law limit (µg/ml) 2.5-12.5 µg/ml 3. Regression equation a)Slope b)Intercept 0.0673 4. Correlation coefficient(r2) 0.9983 5. Molar absorptivity 35958.61 6. Precision 99.83, 98.87 7. Specificity A 10 µg/ml solution of candidate drug in solvent(methanol and distilled water in ratio of 90:10 respectively) at UV detection of 230.5nm will show an absorbances value of 0.4583 0.2386 4: CONCLUSION: The Vierodt’s method permits simple, rapid and direct determination of Phenylephrine hydrochloride and Levocetirine dihydrochloride is commercially available tablet dosage form without previous separation.The results of analysis of two drugs from tablet formulation using method was found close to 100%, standard deviation was satisfactorily low indicating which showed that there is no interference of excipients. 5. ACKNOWLEDGMENT: The authors wish to thank Zim Laboratories, Nagpur, Maharastra for providing the gift samples of Phenylephrine HCl and Levocetirizine dihydrochloride. 6. REFERENCES: 1. Budhavari S. The Merk Index. 14th ed., Merk Research Lab. Division of Merck and Co. Inc. Whitehouse station NJ; 2001. p. 1011. 2. Sweetman SC, Martin D. The Complete Drug Reference. 35th ed. Pharmaceutical Press, London; 2007. p. 526. 3. Budhavari S. The Merk Index. 14th ed. Merk Research Lab. Division of Merck and Co. Inc. Whitehouse station NJ; 2001. p. 1307. 4. Maithani M, Raturi R, Gautam V, Singh R. Develpoment and Validation of a RPHPLC method or the determination of Chlorpheniramine maleate and Phenylephrine in pharmaceutical dosage form.International Journal of Comprehensive Pharmacy 2010;1(05):11-12. 5. Joshi S, Bhatia C, Singh CB, Singh MR. A validated HPLC method for simultaneous estimation of Phenylephrine hydrochloride, Guaiphenesine,Ambroxol hydrochloride and Levoceterizine hydrochloride in counter cough formulation.Journal of the Indian Chemical Society 2010;87(11):1425-1429. 6. Selvan PS, Gopinath R, Saravanan VS, Gopal N. Simultaneous estimation of Levocetrizine, Ambroxol, Phenylpropanolamine and and Paracetamol in combined dosage form by RP-HPLC method. Indian J Chem 2006;18(14):2591-96. 7. Birajdar AS, Meyyanathan SN, Raja RB, Krishnaveni , Suresh B. Simultaneous analysis of Ambroxol HCl with Cetrizine HCl and Levocetrizine dihydrochloride in solid dosage form by RP-HPLC. Acta Chromatgra 2008;20(8):411-21. 8. Ashokkumar S, Senthil Raja M, Perumal P. RP-HPLC Method Development and Validation for Simultaneous Estimation of Montelukast Sodium and Levocetrizine. Journal of Pharmaceutical Research 2009; 1(4): 8-12. 9. Dhaneshwar SR, Bhusari VK. Validated simultaneous quantitation of Levocetrizine hydrochloride and Nimesulide in bulk drug and formulation. International Journal of Comprehensive Pharmacy 2011; 2(02):67. 10. Merukar SS, Mhaskar PS, Bavaskar SR, Burade KB, Dhabale PN. Simultaneous spectrophotometric methods for estimation of Levocetrizine and Pseudoephidrine in Pharmaceutical Tablet Dosage Form. Journal of Pharmaceutical Sciences and Research 2009;1(2):3842. 11. Sawant R, Joshi R, Lanke P, Bhangale L. Spectrophotometric method for simultaneous estimation of Paracetamol, Phenyleprine hydrochloride and Chlorpheneramine maleate in tablet dosage form. JPRHC 2010;3(2):23-28. 12. Choudhari V, Kale A, Kuchekar B, Gawli V, Patil N. Simultaneous determination of Montelukast sodium and Levocveterizine dihydrochloride in pharmaceutical preparations by Ratio Derivative Spectroscopy. International Journal of Pharm Tech Research March 2010;2(4):985-987. 13. Indian Pharmacopoiea. Government of India Ministry of Health and Family Welfare;2007. Vol.2. p. 893. 14. British Pharmacopoeia. Int Ed.Published on the recommendation of the medicine commission pursuant tomedicine act 1968; 1993.vol.1. p. 509. 15. Shende P, Shah V, Ghodke D. Validation of UV Spectrophotometric method for estimatiion of Levocetrizine dihydrochloride in bulk and pharmaceutical formulation. Journal of Pharmacy 2010; 3(10):2386-2387. 16. Prabhu SL, Shiwankar AA, Kumar CD. Simultaneous UV spectrophotometric estimation of Ambroxol hydrochloride and Levocetrizine dihydrochloride. Indian Journal of Pharmaceutical Science 2008;70(2):236-8.