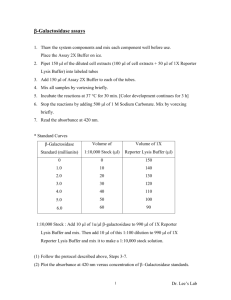
Materials and methods
advertisement

Supplementary Information Materials and methods Cell culture, reagents and tissue samples SW48, DLD-1, HCT 116, and HT-29 (ATCC) were grown in DMEM with 10% FBS. COLO 201 (ATCC) was grown in DMEM with 10% FBS and 1 mM sodium pyruvate. Caco-2, LS 174T and CCD-18Co (ATCC) were grown in MEM with 10% FBS and 1 mM sodium pyruvate. K-562 (ATCC) was grown in DMEM with 10% FBS and 3 mM sodium pyruvate. FHC (ATCC) was grown in the growth medium according to ATCC. Polyclonal antibodies including anti-CHK antibody Lsk (C-20), anti-Src antibody Src2, normal polyclonal IgG and anti-Csk (C-20) were from Santa Cruz. Polyclonal anti-CHK antibodies CHK (C89) and Matk (N20) were raised against synthetic peptides and purified using peptide-affinity columns (the epitope sequences are listed in supplementary Figure 2). Anti-Src monoclonal antibodies MAb2-17 and MAb327 were from Quality Biotechnology and from a hybridoma provided by Dr. Joan Brugge, respectively. Polyclonal phospho-Src antibodies anti-Y530-P and anti-Y419-P were from Cell Signaling. Monoclonal anti-tubulin antibody (Ab-1) was from Oncogene Sciences. Monoclonal anti-GAPDH antibody was from IMGENEX. Human colon cancer tissues and adjacent normal colon tissues from 6 patients were obtained from Alberta Research Tumor Bank and the quality of cancers were further verified by histopathological examination of the samples. 1 Cell lysis in RIPA lysis buffer and western blotting Subconfluent cells in tissue culture dishes were rinsed twice with cold PBS. Cells were then lysed on ice for 10 min in lysis buffer, which is RIPA buffer (50 mM Tris-HCl, pH 7.2, 0.15 M NaCl, 1.0 mM EDTA, 0.1% SDS, 1.0% Triton X-100, 1.0% sodium deoxycholate) freshly supplemented with phosphatase and protease inhibitors (1 mM sodium orthovanadate, 7.5 mg/ml p-nitrophenolphosphate, 50 g/ml leupeptin, and 10 g/ml aprotinin). After 20 passes through 21-gauge needles, lysates were clarified by centrifugation at 10,000 g for 20 min. Proteins were quantified using the Bradford assay, and lysates were either used immediately or stored at -80°C. Colon tissues were rinsed with PBS and homogenized in RIPA lysis buffer on ice using a Dounce Homogenizer and clarified by centrifugation. Proteins were resolved by SDS-PAGE and transferred to membranes. Antibodies for CHK included CHK (C89) (1 g/ml), Matk (N20) (2 g/ml) and Lsk (C-20) (1 g/ml). Anti-Csk (1:200), anti-tubulin (0.5 g/ml), MAb327 (1 g/ml), Src2 (1 g/ml), anti-Y419-P (1:1000) and anti-Y530-P (1:1000) were also used for western blotting. Proteins were visualized by exposure to film following detection using enhanced chemiluminescence (ECL Plus, Amersham), according to manufacturer’s instructions. Quantification of protein bands was done by using ImageQuant software on a Storm 860 phosphorImager from Molecular Dynamics. RT-PCR Total RNA from mouse tissues (stabilized in RNAlater reagent, QIAGEN) and cultured cells were isolated using RNeasy (QIAGEN) with individual protocols for brain, muscle, 2 and tissues, and cultured cells respectively. PAGE-purified primer sets used to amplify mouse CHK splicing variants 1 and 2 were as follows (Figure 2a): sense, 5'CCTCCGAGGGTCGCGCCTAAGCAG-3'; antisense, 5'- AGAGTCAGATGCTGCAGGTCGAGTA-3', with predicted products of 650 bp and 798 bp respectively. For splicing variant 3, the sense primer was 5'- CAAAGAGCCGCAGCATCTGACC-3', with a predicted product of 834 bp. Primers for all human CHK splicing variants were as follows (Figure 3c): sense, 5'TACCGCGTCAAGCACCACACCAG-3'; CACTCTCCACTCTCTCGGTCCTCTG-3'. antisense, PCR products 5'were analyzed electrophoretically and the ethidium bromide stained gels were photographed under UV light. Cell lysis in NP-40 lysis buffer, Src kinase assay and co-immunoprecipitation Cells were lysed as in the RIPA lysis procedures except replacing RIPA lysis buffer with NP-40 lysis buffer (50 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 1.0 mM EDTA, 1% NP-40) freshly supplemented with phosphatase and protease inhibitors, and lysing for 30 min on ice with vortexing at 10 minute intervals. Cell lysates were incubated with an excess of Src antibody MAb327 (0.5 g antibody/100 g cell extract) for 1 h on ice, followed by addition of 30 l of 25% protein G-agarose and 200 l NP-40 lysis buffer and rotation at 4 °C for 1 h. Mouse IgG was used as control. The immune complexes were washed with NP-40 lysis buffer and with kinase assay buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM DTT, 5 mM MgCl 2, 200 M sodium orthovanadate and 4 mg/ml p-nitrophenolphosphate). Src kinase activity 3 was measured in the immmunoprecipitates by the addition of 50 l of kinase assay buffer containing 0.02 mM ATP, 10 Ci of [-32P] ATP (3000 Ci/mmol), and 100 M Src optimal peptide. Following incubation at 30 °C for 30 min, 25 l of 50% (v/v) acetic acid was added to each tube, after which 50 l was spotted onto P81 phosphocelluose paper. The filter papers were then washed four times with 0.5% phosphoric acid to remove free ATP, rinsed once with acetone, dried, and counted in a scintillation counter. Src immunoprecipitates described above, as well as CHK immunoprecipitates using Lsk (C-20), were also analyzed by western blotting to examine coimmunoprecipitation of CHK and Src. DNA Transfection 50% confluent DLD-1 cells were transiently transfected with pcDNA3 (Invitrogen), pcDNA3-CHK (Chong et al., 2004) or EGFP C1 (Clontech) plasmid using Lipofectamine 2000 (Invitrogen) as per the manufacturer’s instructions except replacing Opti-MEM I Reduced Serum Medium with DMEM. 24 h later, cells were lysed with NP40 lysis buffer and subjected to western blotting, immunoprecipitation and Src kinase assay. Transfection of siRNA siRNA duplexes of human CHK (sense, 5'-GCACCCAGUGUAUCACCAAdTdT-3'; antisense, 5'-UUGGUGAUACACUGGGUGCdTdT-3') and human Csk (sense, 5'GCAUCAUCCCAGCCAACUAdTdT-3'; antisense, 5'- UAGUUGGCUGGGAUGAUGCdTdT-3') were designed using BLOCK-iT RNA 4 Designer program (Invitrogen) as the top-rank siRNA sequences, and then the singlestranded sequences were synthesized in the RNA facility of University of Calgary. siRNA duplexes of negative control (sense, 5'-UUCUCCGAACGUGUCACGUdTdT-3'; antisense, 5'-ACGUGACACGUUCGGAGAAdTdT-3') were designed by QIAGEN, and the single-stranded sequences were synthesized in the RNA facility of University of Calgary. Alexa488-labelled control siRNA duplex was from QIAGEN. Double-stranded siRNA was prepared by heating the complementary single strands together at 90ºC for 1 min, then 37ºC for 1 h. Cells grown in dishes at approximately 50% confluence were transiently transfected with siRNA (CHK, Csk, control, or Alexa488-control), using siLentFect lipid reagent (BIO-RAD) as per the manufacturer’s instructions. Transfections and siRNA concentrations are: 1, Control siRNA (20 nM); 2, CHK siRNA (20 nM); 3, Csk siRNA (20 nM); 4, CHK siRNA (10 nM) + Csk siRNA (10 nM). Cells were lysed 36 h later using NP-40 lysis buffer for western blotting and Src kinase assay. Immunofluresence Cells grown on coverslips were fixed in formaldehyde and permeabilized (each step was 10 min at room temperature, sequentially in PBS containing 3.7% formaldehyde, 0.5% Triton X-100, and 0.05% Tween 20), then incubated with 1 g/ml antibody (Lsk (C-20), Mab327) in PBS containing 1% BSA for 45 min at 37ºC in a humidified chamber. After washing 5 times in PBS containing 0.05% Tween 20, the cells were incubated with secondary antibody for 45 min at 37ºC in the chamber. 2mg/ml Alexa Fluor488 chicken anti-mouse IgG (H+L) from Molecular Probes (Eugene, OR) was diluted 500 fold, and 5 1.5mg/ml Cy3-conjugated AffiniPure Donkey Anti-Rabbit IgG (H+L) from Jackson Immunoresearch Laboratories (West Grove, PA) was diluted 100 fold in PBS containing 1% BSA. After immunostaining, the coverslips were washed 5 times with PBS, stained with DAPI (Sigma, 1 g/ml in PBS), washed again and mounted with antifade reagent (Molecular Probes). Specimens were observed and digital images were acquired with a Zeiss-Axioskop microscope. Images were deconvoluted using ImageJ software. Soft agar colony assay 2 × 103 trypsinized and separated DLD-1 cells were added to 3 ml of DMEM medium containing 10% FBS, antibiotics, and 0.3% agarose. After vortexing briefly, the cells were plated on 60 mm dishes over a 3 ml bottom layer of pre-hardened 0.5% agarose medium. Additional 0.3% agarose medium was added every 3 days. The cells were maintained at 37°C in a 5% CO2 incubator for 10 days and the colonies containing over 100 cells were counted with an inverted microscope. 6