Practice Problems:
advertisement

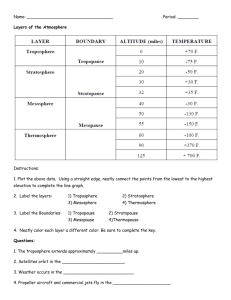
Practice Problems: For 9/16/09 From the book: Chapter 1: 4, 6, 9, 21, 25, 28, 32, 40, 44, 46, 49, 51, 56. Appendix E of the text has answers to even numbered questions 9. Anaerobic bacteria are able to metabolize carbon without the use of oxygen, and usually cannot live in an oxygen environment. 21. Producers are organisms that provide their own food, usually through photosynthesis. Consumers must consume other organisms as a food source. 25. The First Law of Thermodynamics states that the energy of the universe is conserved. An example of an energy transformation would be burning hydrocarbons. Chemical energy is transformed into thermal energy. 44. a. b. 1L H 2O 1000g 10g NH4 1mol 5.6 x104 M 6 1L 10 g H 2 O 18g 5.4x10 -6 mol CaCO3 40.08g 1 L 10 6 0.22ppm 1L 1mol 1000g 49. a. The ocean acts a sink for atmospheric carbon dioxide. The ocean’s buffer capacity means that it is able to uptake a large amount of CO2 from the atmosphere. Carbonaceous sediments form on the ocean floor. These sediments are a long lived reservoir for carbon. b. Limestone makes up a long-term reservoir for carbon. Limestone is formed in the oceans, and is broken down when plate tectonics exposes the limestone to chemical weathering . c. Crude oil is made up of decayed biological materials. In the earth’s crust, crude oil represents a long-lived reservoir for carbon. 51. Nitrogen fixation is the process that makes atmospheric nitrogen available to plants. a. The bacteria that bring about this process can be found in soils or attached to the roots of certain plants. b. These bacteria initially produce ammonia. Other problems: Human activities can have a major influence on which of the following compounds in the atmosphere on time scales of a few years? Explain your reasoning for each compound. O2, CO2, N2. In the short-term, human activities can only have a major effect on the atmospheric concentration of CO2. The lifetime of carbon in the terrestrial biosphere/atmosphere/surface ocean system is on the order of 70 years, by changing the flux between any of these reservoirs, or by changing the inputs of carbon into any of these reservoirs, we can have an influence on the atmospheric concentrations. O2 and N2 are controlled by processes that occur over millions of years. It is not probable that human activities could affect the atmospheric concentrations of these gases over ‘short’ time scales. Why does the ocean resist changes in pH? The oceans have a HCO3-/CO32- buffer system that allows the uptake of acid (CO2) without changing the pH very much. Using the pre-industrial carbon cycle figure presented in class, calculate the lifetime of carbon in the (atmosphere + terrestrial biosphere + soil) reservoir relative to uptake by the ocean. Referring to the figure: (2000+730+615)TgC / 60TgC/yr = 56yrs. Is the following statement reasonable? Reducing atmospheric CO2 concentrations by planting more trees is not effective because trees are a short-term reservoir for carbon. To some degree this statement is true. It is possible to sequester carbon in the terrestrial biosphere, however, depending on the specific ecosystem, the lifetime of the reservoir may be anywhere from 1 year to a maximum of several thousand years. These reservoirs would have to be well-managed. One fire could put all of the sequestered carbon back in the atmosphere in a very short time. A developer wants to raise atmospheric O2 concentrations by planting managed forests that will be cut down every couple decades, sealed in weighted leak-proof containers, and dropped into the deep ocean. Will this plan work? Explain. No, this plan would not work. Raising atmospheric O2 levels by converting atmospheric CO2 to O2 through photosynthesis is limited by the amount of CO2 in the atmosphere. Using this method, one could only raise O2 levels by a theoretical maximum of 370ppmv. This is almost nothing relative to the 20% O2 currently found in the atmosphere. For 9/16/09 From the book: Chapter 3: 3, 5, 8. Chapter 4: 5, 10, 11, 12, 31, 37, 39, 40, 41. Appendix E of the text has answers to even numbered questions Chapter 3 3. The pressure of the atmosphere drops off exponentially with altitude (P=Poe-z/H). The pressure at sea level is approximately 1000mbar. The pressure at the tropopause is approximately 200mbar. Only 20% of the mass of the Earth’s atmosphere is contained in the stratosphere. 5. The amount of water vapor in the Earth’s atmosphere generally lies between 1 and 3% Chapter 4: 5. A free radical has one unpaired electron. A hydroxyl radical is a free radical; it has a neutral charge. Hydroxide ion has a full octet and has a negative charge. 11. The Lewis electron structure for NO: .. .. : N - O. .. .. There is one unpaired electron because NO is a radical compound. 31. Tall smokestacks release pollution into better mixed air, and commonly above the nighttime boundary layer. This means that the pollution is dispersed better than if it were released near the ground. All else being equal, it is good to disperse a pollutant rather than have it concentrated in one area. However, the best thing would be to reduce emissions overall. 37. NO2 h NO O O O2 M O3 M NO2 + H2O to make 2OH, as the book describes it, does not really happen. Additional OH, in addition to the photolysis of O3, can be produced in the troposphere through the photolysis of formaldehyde. HCHO h H HCO H 2 CO HCHO OH HCO H 2 O HCO O 2 HO 2 CO HO2 is produced without using up OH. 39. OH NO2 HNO 3 HO 2 HO 2 H 2 O 2 O 2 OH CO, or CH 4 , or VOC 41. j1 O3 h O 2 O(1 D); 320nm k2 O(1 D) H 2O 2OH Other Problems: Consider xenon, an un-reactive atmospheric gas whose mixing ratio is 900pptv. What is the concentration of xenon at 1010 mbar (sea level) and at 200 mbar (tropopause)? Assume the temperature of the tropopause is 190K. Use PV=nRT At 1010 mbar, assume T = 300K n PV (1atm)(0.00 1L) 900 4.06 x10 5 moles 12 3.66 x10 14 mol Xe -1 -1 RT (0.08206L atm mol K )(300K) 10 6.022x10 23 molec 3.66 x10 14 mol Xe 1 mol 2.2 x1010 molecules cm -3 At 200 mbar, assume T = 190K n PV (0.2atm)(0. 001L) 900 1.28 x10 5 moles 12 1.15 x10 14 mol Xe -1 -1 RT (0.08206L atm mol K )(190K) 10 6.022x10 23 molec 1.15 x10 14 mol Xe 1 mol 7.0 x109 molecules cm -3 Why is the troposphere well-mixed, but the stratosphere is not? The troposphere is heated form the Earth’s surface. Turbulent convection mixes tropospheric air vertically on time scales of a month. The stratosphere is heated from above due the photodissociation of O2. This temperature inversion leads to very stable stratification of the air in the stratosphere. How can it be that the lifetime of air in the troposphere with respect to transport to the stratosphere is 7.4 years, but the lifetime of air in the stratosphere with respect to transfer to the troposphere is 1.3 years? This is possible because the stratosphere only contains 20% of the mass of the atmosphere. Even though the mass of air exchanged between the troposphere and stratosphere is the same (conservation of mass). To calculate a lifetime, we divide the mass in the reservoir by the mass transported. In the case of the troposphere and stratosphere, the mass of the stratosphere is 20% the mass of the atmosphere, and the troposphere is 80% the mass of the atmosphere. What factors affect the production of OH in the troposphere? Ozone concentration, water vapor concentration, pressure, and the actinic flux of light less than 320nm, all affect the production of OH in the troposphere. It seems that you need to have ozone to make ozone. Where does the initial ozone come from? The initial ozone would have been transported from the stratosphere. Explain how NOx and HOx act as catalysts for ozone production in the troposphere. NOx cycles between NO and NO2. Each time NO2 is formed without the oxidation of NO by O3, there is the potential for new ozone formation. This usually occurs by the oxidation of NO by HO2. HOx cycles between OH and HO2. OH oxidizes reduced carbon in the atmosphere. The product of this reaction is peroxy radicals and HO2. HO2 (and peroxy radicals) facilitate the production of ozone by oxidizing NO to NO2. The oxidation of NO also represents the reduction of HO2 back to OH; therefore, completing the catalytic cycle. The overall lifetime of CH3CCl3 (methyl chloroform) in the atmosphere is 5.7 years and is controlled by oceanic uptake (40 years), transport to the stratosphere (30 years), and reaction with OH. Calculate the lifetime of CH3CCl3 due to reaction with OH in the troposphere. Calculate the concentration of OH if the rate constant for the reaction between CH3CCl3 and OH is 6.7x10-15 cm3 molecules-1 s-1. Lifetime of CH3CCl3 with respect to OH: 1 1 1 1 1 1 1 1 1 1 1 1 OH 8.5years tot ocean strat OH OH tot ocean strat OH 5.7 40 30 Concentration of OH; 8.5years = 2.7x108s 1 1 1 [OH] [OH] 5.6x10 5 molecules cm -3 -15 3 k[OH] k 6.7x10 cm molecules 1s 1 (2.7 x108 s)