Guidelines for Research with Adenovirus
advertisement

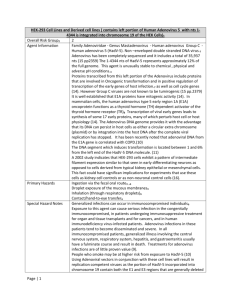
INSTITUTIONAL BIOSAFETY COMMITTEE SAN DIEGO STATE UNIVERSITY GUIDELINES FOR RESEARCH WITH ADENOVIRUS I.Introduction This document provides essential information for Research Investigators regarding work with human Adenovirus, Adenovirus vector systems and Adeno-Associated Virus (AAV) and vector systems in the lab and in animal models. The IBC will determine the final Biosafety Level (BSL) and/or Animal Biosafety Level (ABSL) for work with Adenovirus, Adenovirus vector system, and AAV based on risk assessment and in accordance with the CDC/NIH Biosafety in Microbiological and Biomedical Laboratories (BMBL), NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines), and this SDSU IBC Guidelines for Research with Adenovirus. Specific requirements within these guidelines will be explicitly indicated in the BUA Approval Letter. Use the requirements specified in the BUA Approval Letter and indicated in the BUA Application as a training tool to inform all research personnel of the procedures for working with Adenovirus, Adenovirus vector system, and/or AAV and AAV vector systems in the research laboratory or animal facility. II.Adenovirus Adenovirus is a human pathogen with 8 serotypes: 1, 2, 3, 4, 5, 7, 40 and 41 and classified in the NIH Guidelines as a Risk Group 2. Adenoviruses most commonly causes respiratory illness; however, depending on the infecting serotype, they may also cause various other illnesses, such as gastroenteritis, conjunctivitis, cystitis (bladder infection), and rash illness. While occupational exposure to Adenovirus is generally through aerosols, fomite contact, fecal-oral route and percutaneous exposure are also exposure routes. Adenovirus is susceptible to some disinfectants (Section IX.), but if work surfaces are not correctly disinfected the virus can survive for prolonged periods outside of host body, in some cases 8 weeks on environmental surfaces at room temperature. There is a vaccine for Adenovirus serotype 4 and Adenovirus serotype 7. The SDSU Institutional Biosafety Committee (IBC) will determine the BSL of work involving Adenovirus based on risk assessment. IBC has determined that work with any Adenovirus serotypes at a minimum require BSL 2 facility and containment PLUS precautions specific to work with adenovirus (BSL-2 + Adeno) as described in Section V of this document. Requirements for BSL 2 facility and work practices are indicated in the BMBL Section IV – Laboratory Biosafety Level Criteria. III.Adenovirus Vectors The SDSU Institutional Biosafety Committee (IBC) will determine the BSL of work involving the use of Adenoviral vectors based on NIH Guidelines and risk assessment. The risk assessment will evaluate the potential for generation of replication/competent adenovirus (RCL), aerosolizing procedures and the potential for oncogenesis from the transgene among other risk factors. Work with adenovirus in the vector form will be at a minimum BSL 2 facility and work practices PLUS precautions specific to work with Adenovirus (BSL-2 + Adeno) as described in Section V of this document. Requirements for BSL 2 facilities and work practices are indicated in BMBL Section IV – Laboratory Biosafety Level Criteria and NIH Guidelines Appendix G. IV.Adeno-Associated Virus Adeno-Associated viruses are in the family of viruses Parvoviridae and are considered to be nonpathogenic to humans even though the virus will integrate in the host genome. AAV serotypes 1-4 are classified by the NIH as Risk Group 1. Work with AAV serotypes 1-4 can be conducted at BSL 1. Work with other AAV serotypes will be at a minimum BSL 2 facility and work practices unless investigator petitions the containment level be downgraded. Using AAV in conjunction with EHS, SDSU 1/4 Rev 07/11 Adenovirus or other helper viruses will be at a minimum BSL 2 facility PLUS precautions specific to work with adenovirus (BSL-2 + Adeno) as described in Section V of this document. Requirements for BSL 2 facilities and work practices are indicated in BMBL Section IV – Laboratory Biosafety Level Criteria and NIH Guidelines Appendix G. V.Laboratory Practices, Containment Equipment (BSL 2 + Adeno) In addition to complying with the corresponding facility and work practice containment as specified in the BMBL Section IV – Laboratory Biosafety Level Criteria and if applicable, NIH Guidelines, work practices and containment for Adenovirus, Adenoviral vectors and AAV work designated at BSL 2 + Adeno requires the following: All vacuum lines must be fitted with a HEPA filter (e.g. “Vacushield J” in-line hydrophobic filter, Product #4402 from Gelman Sciences). No work with Adenovirus is permitted on the open bench. A Biosafety Cabinet must be used for all manipulations including (but not limited to): o pipetting o harvesting infected cells for RNA/DNA/proteins o loading and opening containers initial delivery of vector in animal hosts Reduce risk of exposure by reducing potential of replication competent adenovirus vector using one of the following methods: o 1st generation vector – deletion of E1 or E1/E3 o 2nd generation vector – deletion of E1, E3, E4 o 3rd generation vector – all viral gene deleted; only essential cis-acting sequences retained BSL 2 + Adeno includes the use of the following personal protective equipment to reduce the potential for mucosal exposure, splash to the face, and exposure of hands: o Gloves o Wrap around outer clothing when introducing vector into animals or performing necropsies. Labcoats are adequate for tissue culture manipulations. o Goggles (not to be confused with safety glasses) o N-95 Respirator, to be used with concentrated titers and highly aerosolizing procedures outside of the Biological Safety Cabinet (contact EH&S for further information) or in ASBL 2 Facility. VI.Animal Research with Adenovirus When animals are infected with adenovirus/adenoviral vectors and some AAV, an Animal BSL2+Adeno (ABSL-2+Adeno) area must be approved and used for the procedure. Concurrent approvals are needed from the Institutional Biosafety Committee (IBC) and the Institutional Animal Care and Use Committee (IACUC). All necropsy must be performed in a necropsy room using Animal BSL-2 + Adenovirus precautionary practices and procedures. Requirements for ABSL-2 containment facility and work practices are indicated in BMBL Section V. Vertebrate Animal Biosafety Level Criteria for Vivarium Research Facilities. Infected animals may excrete adenovirus (especially in the first 72 hours after infection). Precautions must be taken not to create aerosols when emptying animal waste material and when washing down cages, or cleaning the room with pressure hoses. It is strongly recommended by the Institutional Biosafety Committee that the lab personnel be responsible for all animal husbandry practices during the first 72 hours at ABSL-2+Adeno following infection of the animal. After 72 hours, the animals can be housed in an ABSL-1 facility. Contact EH&S for Standard Operating Procedure for work with ABLS-2+Adeno. Special training must be given to all animal husbandry personnel on adenovirus, the hazards associated with the work, required practices and procedures and proper handling of bedding, cage EHS, SDSU 2/4 Rev 07/11 washing, and all other husbandry materials associated with the experiment. Contact EH&S at (619) 594-2865 to schedule a group training. VII.Precautions Adenovirus is a pathogen of respiratory and gastrointestinal mucous and eye membranes, and does not have to be replication-competent to cause corneal and conjunctival damage. Goggles must be worn when working with the agent/vector. The replication-defective virus may be complemented in vivo thereby causing the vector to become replication competent. Adenovirus (unlike HIV or herpes), is quite stable. After having been extracted with ether, and/or chloroform, it can still be infective. Signs and labels must be placed to indicate each area where Adenovirus is used or stored including, but not limited to, Biosafety cabinets, incubators, refrigerators, laboratory entrance doors, etc. VIII.Employee Exposure 1. Eye or gastrointestinal mucous membrane exposure from splash or aerosols – rinse a minimum of 15 minutes in eye wash or flush area with water and take patient immediately to Sharp ReesStealy La Mesa. Notify prior to seeking medical attention the lab manager/principal investigator, biosafety officer or Human Resources. 2. Inhalation exposure from aerosols – take patient immediately to Sharp Rees-Stealy La Mesa. Notify prior to seeking medical attention the lab manager/principal investigator, biosafety officer or Human Resources. 3. Needlestick and/or Sharps Exposure – to prevent inoculation, take patient immediately to Sharp Rees-Stealy La Mesa. Notify prior to seeking medical attention the lab manager/principal investigator, biosafety officer or Human Resources. SYMPTOMATOLOGY: Acute respiratory illness (cold-like symptoms); pneumonia. Conjunctival infection (or red eye); corneal inflammation. Contact Information Sharp Rees Stealy Medical Centers Occupational Health Services 5525 Grossmont Center Drive La Mesa, CA 91942 (619) 644-6600 IX.Decontamination The most effective germicides (with minimum 15 minute contact time) are: 1% Sodium hypochlorite 2% Glutaraldehyde 0.25% Sodium Dodecyl Sulfate X.Employee Right-to-Know It is important that all lab personnel (even those not directly working with the virus) be informed and aware that Adenovirus is being used in the lab. As the Principal Investigator your signature below indicates that you agree to comply with these requirements and will acknowledge and accept responsibility for ensuring compliance including all individuals who enter or work in your laboratory or collaborate in carrying out your research. Although you may choose to delegate aspects of the biosafety program in your laboratory to other EHS, SDSU 3/4 Rev 07/11 laboratory staff, you are ultimately responsible for all activities occurring in your laboratory. Contact Environmental Health and Safety, Institutional Biosafety Officer at (619) 594-2865 with questions related these responsibilities. PI Name: Signature: Date: Return this form to Graduate and Research Affairs, Division of Research Affairs, Student Services East, Room 1410, MC 8220, retain a copy for your reference. I have attended laboratory specific safety training for this standard operating procedure. I have read and understood this standard operating procedure and I have had my questions answered. Date EHS, SDSU Attendee Name (Print) 4/4 Signature Rev 07/11