Clinical-Trial-List
advertisement

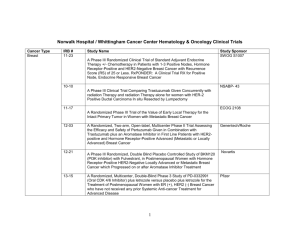
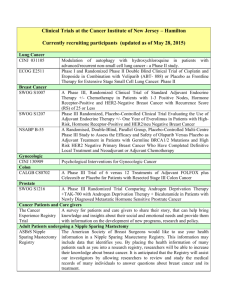
Norwalk Hospital / Whittingham Cancer Center Hematology & Oncology Clinical Trials Cancer Type Breast IRB # 11-23 Study Name Study Sponsor SWOG S1007 A Phase III Randomized Clinical Trial of Standard Adjuvant Endocrine Therapy +/- Chemotherapy in Patients with 1-3 Positive Nodes, Hormone Receptor-Positive and HER2-Negative Breast Cancer with Recurrence Score (RS) of 25 or Less. RxPONDER: A Clinical Trial RX for Positive Node, Endocrine Responsive Breast Cancer 10-10 NSABP- 43 A Phase III Clinical Trial Comparing Trastuzumab Given Concurrently with radiation Therapy and radiation Therapy alone for women with HER-2 Positive Ductal Carcinoma In situ Resected by Lumpectomy 11-17 ECOG 2108 A Randomized Phase III Trial of the Value of Early Local Therapy for the Intact Primary Tumor in Women with Metastatic Breast Cancer 12-03 12-21 13-15 A Randomized, Two-arm, Open-label, Multicenter Phase II Trial Assessing the Efficacy and Safety of Pertuzumab Given in Combination with Trastuzumab plus an Aromatase Inhibitor in First Line Patients with HER2positive and Hormone Receptor-Positive Advanced (Metastatic or Locally Advanced) Breast Cancer A Phase III Randomized, Double Blind Placebo Controlled Study of BKM120 (PI3K inhibitor) with Fulvestrant, in Postmenopausal Women with Hormone Receptor-Positive HER2-Negative Locally Advanced or Metastatic Breast Cancer which Progressed on or after Aromatase Inhibitor Treatment A Randomized, Multicenter, Double-Blind Phase 3 Study of PD-0332991 (Oral CDK 4/6 Inhibitor) plus letrozole versus placebo plus letrozole for the Treatment of Postmenopausal Women with ER (+), HER2 (-) Breast Cancer who have not received any prior Systemic Anti-cancer Treatment for Advanced Disease 1 Genentech/Roche Novartis Pfizer Colon 14-12 A Randomized, Double-blind, Vehicle-controlled, Parallel-group Phase 2 Study of the Efficacy, Safety, Pharmacokinetics, and Pharmacodynamics of RTA 408 Lotion in the Treatment of Patients at Risk for Radiation Dermatitis Reata 12-14 A Phase II/III trial of Neoadjuvant FOLFOX, with Selective Use of Combined Modality Chemoradiation versus Preoperative Combined Modality Chemoradiation for Locally Advanced Rectal Cancer Patients Undergoing Low Anterior Resection with Total Mesorectal Excision Alliance N1048 10-20 A Phase III Trial of 6 vs. 12 Treatments of Adjuvant FOLFOX plus Celecoxib or Placebo for Patients with Resected Stage III Colon Cancer CALGB 80702 14-01 An Open-Label, Multi-Center, Randomized Phase 1B/2 Study of PF05212384 (PI3K / mTOR inhibitor) Plus 5-Luorouracil-Leucovorin-Irinotecan (FOLFIRI) Versus Bevacizumab Plus FOLFIRI in Metastatic Colorectal Cancer A Phase 1, Open-label, Multi-center, Dose Escalation Study of the Safety, Tolerability, Pharmacodynamic and Pharmacokinetic Properties of Intravenous GC4419 in Combination with Radiation and Chemotherapy for Squamous Cell Cancers of the Head and Neck Pfizer Head and Neck 13-07 Hematology 12-24 A Phase IIa, Open-Label, Multicenter Study of Single-Agent MOR00208, an Fc-Optimized Anti-CD19 Antibody, in Patients with Relapsed or Refractory B-Cell Non-Hodgkin’s Lymphoma Morphosys 11-26 A Phase III Randomized Study of Oral Sapacitabine in Elderly Patients with Newly Diagnosed Acute Myeloid Leukemia Cyclacel 14-03 A Randomized Phase III Study of Bendamustine Plus Rituximab Versus Ibrutinib Plus Rituximab Versus Ibrutinib Alone in Untreated Older Patients ( ≥ 65 Years of Age) with Chronic Lymphocytic Leukemia (CLL) Alliance A041202 14-07 A phase 3b randomized study of lenalidomide (CC-5013) plus rituximab maintenance therapy followed by lenalidomide single-agent maintenance versus rituximab maintenance in subjects with relapsed/refractory follicular, marginal zone or mantle cell lymphoma Celgene 2 Galera Pancreas 14-09 An International, Multi-Center, Double-Blind, Randomized, Phase III Trial of 90Y-Clivatuzumab Tetraxetan plus Low-Dose Gemcitabine Versus Placebo plus Low-Dose Gemcitabine in Patients with Metastatic (Stage IV) Pancreatic Adenocarcinoma Who Received at Least Two Prior Treatments (PANCRIT-1) Immunomedics Prostate 14-05 Phase III trial of enzalutamide (NSC # 766085) versus enzalutamide, abiraterone and prednisone for castration resistant metastatic prostate cancer Alliance A031201 Bladder 10-21 A Randomized Double-Blinded Phase III Study Comparing Gemcitabine, Cisplatin and Bevacizumab to Gemcitabine, Cisplatin and Placebo in Patients with Advanced Transitional Cell Carcinoma CALGB 90601 Lung 12-09 Decision Impact Analysis of Foundation Medicine’s Next Generation Sequencing Test in Advanced Non-Small Cell Lung Cancer Foundation Medicine 14-10 A Phase II, Open Label, Single-arm Study to Assess the Safety and Efficacy of AZD9291 in Patients with Locally Advanced/Metastatic Non Small Cell Lung Cancer whose Disease has Progressed with Previous Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and whose Tumours are Epidermal Growth Factor Receptor Mutation and T790M Mutation Positive (AURA2) AstraZeneca Solid tumors 13-03 A Prospective Observational Study to Examine, in Routine Clinical Practice in the US, Practice Patterns and Impact on Clinical Decision Making Associated with the FoundationOne™ Next Generation Sequencing (NGS) Test Foundation Medicine Phase 1 13-01 A Phase 1 First-in-Human Study Evaluating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of AMG 232 (p53 inhibitor) in Adult Subjects with Advanced Solid Tumors A Phase I, Open-Label, Multiple-Ascending Dose Trial to Investigate the Safety, Tolerability, Pharmacokinetics, Biological and Clinical Activity of MSB0010718C (anti-PD-L1) in Subjects with Metastatic or Locally Advanced Solid Tumors and Expansion to selected Indications Amgen 14-02 3 EMD Serono Signature (P to P) Pending Quorum Modular phase II study to link targeted therapy to patients with pathway activated tumors: Module 2 - Dovitinib for patients with tumor pathway activations inhibited by dovitinib including tumors with mutations or translocations of FGFR, PDGFR, VEGF, cKIT, FLT3, CSFR1, Trk and RET Novartis Quorum Modular phase II study to link targeted therapy to patients with pathway activated tumors: Module 4 – LGX818 for patients with BRAFV600 mutated tumors Novartis Quorum Modular Phase II Study to Link Targeted Therapy to Patients with Pathway Activated Tumors: Module 1 – BKM120 for Patients with PI3K-activated Tumors Novartis Quorum Modular phase II study to link targeted therapy to patients with pathway activated tumors: Module 3 - MEK162 for patients with RAS/RAF/MEK activated tumors Novartis A Phase III, Open Label, Randomized Study of AZD9291 versus PlatinumBased Doublet Chemotherapy for Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer whose Disease has Progressed with Previous Epidermal Growth Receptor Tyrosine Kinase Inhibitor Therapy and whose Tumours harbour a T790M mutation within the Epidermal Growth Factor Receptor Gene (AURA3) AstraZeneca Updated 7/10/14 4