Graphical Abstract
To create your abstract, type over the instructions in the template box below.
Fonts or abstract dimensions should not be changed or altered.
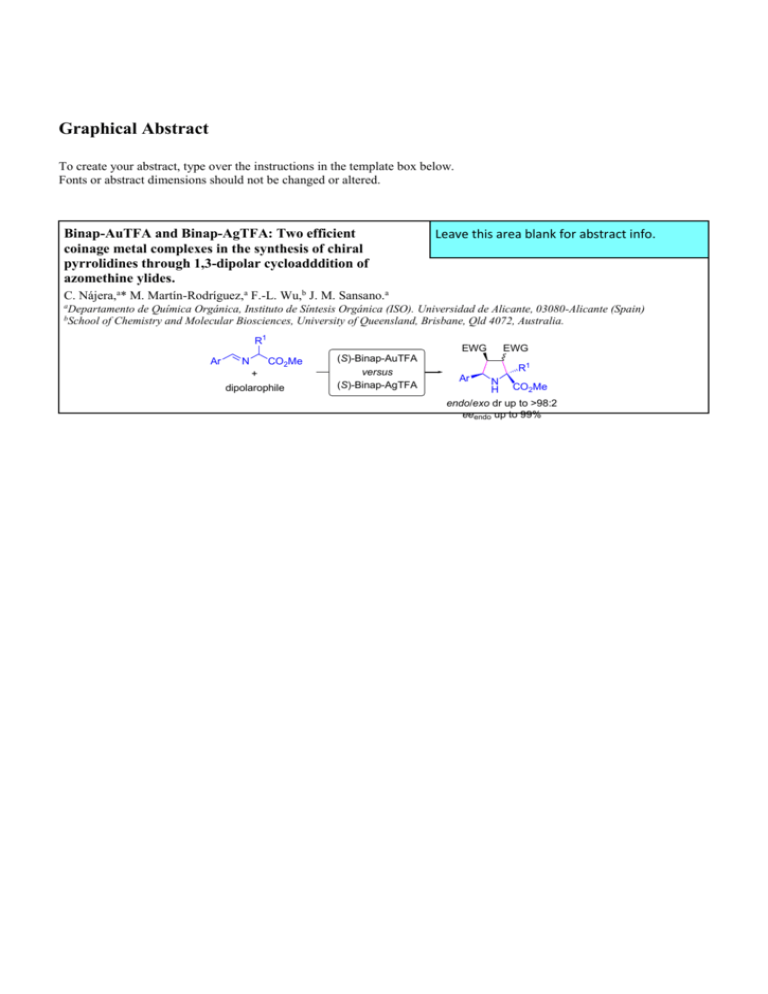
Binap-AuTFA and Binap-AgTFA: Two efficient
coinage metal complexes in the synthesis of chiral
pyrrolidines through 1,3-dipolar cycloadddition of
azomethine ylides.
Leave this area blank for abstract info.
C. Nájera,a* M. Martín-Rodríguez,a F.-L. Wu,b J. M. Sansano.a
aDepartamento
bSchool
de Química Orgánica, Instituto de Síntesis Orgánica (ISO). Universidad de Alicante, 03080-Alicante (Spain)
of Chemistry and Molecular Biosciences, University of Queensland, Brisbane, Qld 4072, Australia.
R1
Ar
N
EWG
CO2Me
+
dipolarophile
(S)-Binap-AuTFA
versus
(S)-Binap-AgTFA
EWG
R1
Ar
N CO Me
2
H
endo/exo dr up to >98:2
eeendo up to 99%
Stereochemistry Abstract
To create your abstract, type over the instructions in the template box below.
Fonts or abstract dimensions should not be changed or altered. You may insert more abstracts by copying this box or by using
the menu option to insert a stereochemistry abstract.
Authors' names here
O
Ph
N
N
H
O
Ee = 99%
[a]D = + 90.4º (c = 1, CHCl3, 99% ee from HPLC)
Source of chirality: (Sa)-Binap-AuTFA
CO2Me
C15H16N2O4
(1S,3R,3aS,6aR)-Methyl 5-methyl-4,6-dioxo-3-phenyl-octahydropyrrolo[3,4-c]pyrrole-1-carboxylate
Authors' names here
O
MeO
N
N
H
O
CO2Me
Ee = 99%
[a]D = +114.0 (c = 0.5, CHCl3, 99% ee from HPLC)
Source of chirality: (Sa)-Binap-AuTFA
C16H18N2O4
(1S,3R,3aS,6aR)-Methyl 3-(4-methoxyphenyl)-5-methyl-4,6-dioxo-octahydropyrrolo[3,4-c]pyrrole-1-carboxylate
Tetrahedron: Asymmetry
Authors' names here
N
O
O
CO2Me
Ph
N
H
Ph
Ee = 99%
[a]D = _74.2º (c = 0.8, CHCl3, 98% ee from HPLC)
Source of chirality: (Sa)-Binap-AuTFA
C22H22N2O4
(1S,3R,3aS,6aR)-Methyl 5-methyl-4,6-dioxo-3-phenyl-octahydropyrrolo[3,4-c]pyrrole-1-carboxylate
Authors' names here
PhO2S
N
SO2Ph
CO2Me
N
H
Ee = 99%
[a]D = _63.9º (c = 1.2, CH2Cl2, 99% ee from HPLC)
Source of chirality: (Sa)-Binap-AuTFA
C23H22N2O6S2
(2S,3S,4S,5R)-Methyl 3,4-bis(phenylsulfonyl)-5-(pyridin-3-yl)pyrrolidine-2-carboxylate
Authors' names here
ButO2C
N
S
N
H
CO2Me
Ee = 99%
[a]D = +58.6 (c = 1, CHCl3, 99% ee from HPLC)
Source of chirality: (Sa)-Binap-AuTFA
C18H28N2O4S
(2S,4S,5R)-4-tert-Butyl 2-methyl 2-isobutyl-5-(thiazol-2-yl)pyrrolidine-2,4-dicarboxylate
3
Tetrahedron: Asymmetry
1
TETRAHEDRON:
ASYMMETRY
Pergamon
Binap-AuTFA and Binap-AgTFA: Two efficient coinage metal
complexes in the synthesis of chiral pyrrolidines through 1,3dipolar cycloadddition of azomethine ylides
Carmen Nájera,a* María Martín-Rodríguez,a Feng-Liu Wu,b José M. Sansanoa
aDepartamento
de Química Orgánica, Instituto de Síntesis Orgánica (ISO). Universidad de Alicante, 03080-Alicante (Spain)
of Chemistry and Molecular Biosciences, University of Queensland, Brisbane, Qld 4072, Australia.
bSchool
Deticated to Prof. H. B. Kagan on the occasion of his 80 birhtday
Abstract—In this work, a comparison between chiral BINAP-AuTFA- and chiral BINAP-AgTFA-promoted catalytic enantioselective
1,3-dipolar cycloadditions of azomethine ylides and alkenes. Maleimides reacted smoothly in very good yields and enantioselections and
trans-1,2-bis(phenylsulfonyl)ethylene reacted after longer reaction times but using smaller amounts of catalyst loading in order to achieve
the highest enantioselectivities. In spite of the scarce induction of these two complexes when tert-butyl acrylate is used as dipolarophile,
chiral gold(I) catalyst afforded, unexpectedly, an excellent enantioselection in the reaction of the iminoester precursor of key intermediate
in the synthesis of hepatitis C virus inhibitor.
© 2016 Elsevier Science. All rights reserved
Coinage metals attract particular interest from synthetic
organic chemists, because those metals become useful
catalysts for synthesizing the core of many important drugs
containing heterocyclic structures.1 The main features of
these metal complexes are the good chemoselectivity, good
functional group compatibility, stability, traits that are
crucial for application in complex molecular environments.
One of the most representative examples concerns the
synthesis of enantiomerically enriched pyrrolidines2
through the catalytic enantioselective 1,3-dipolar
cycloaddition3 (1,3-DC) between azomethine ylide and
alkenes. In fact, silver(I)4,5 and copper(I)6 catalyzed 1,3DC are very well known7 and constitute the most reliable,
sure and inexpensive enantioselective methodology to built
up to four stereogenic centres of the resulting proline
derivatives, in only one reaction step. In addition, they
exhibit more versatility and wider scope than the analogous
enantioselective
organocatalysed
1,3-dipolar
cycloadditions.3a,8
Chiral gold complexes have been employed in
enantioselective activation of allenes and nucleophilic
additions onto alkynes,9 but they have not so extensively
studied as catalysts in these cycloadditions. Only Toste et
al. reported a very efficient enantioselective cycloaddtion
of münchnones and electron-defficient alkenes employing
(Sa)-Cy-SEGPHOS(AuOBz)2
(3.5
mol%).
This
transformation, followed by an ester/amide formation,
———
furnished pyrrolines with very high enantioselection. 10
*
In this communication, and continuing with the research
line involving the chiral BINAP complex-promoted 1,3DC, we survey the efficiency of (Ra)- or (Sa)-BINAPAuTFA (TFA = trifluoroacetate anion) in the classical
intermolecular 1,3-DC employing iminoesters and
electrophilic alkenes, establishing a direct comparison with
the analogous processes catalyzed by (Ra)- or (Sa)-BinapAgTFA complexes.
One of the best test to prove the ability of a chiral catalyst
in a silver(I) catalysed enantioselective 1,3-dipolar
cycloaddition is the transformation of azomethine ylides
(generated from iminoesters 1) and N-methylmaleimide
(NMM) in enantiomerically enriched prolines 2 (Scheme
1). The chiral gold complexes were prepared in situ from
(Me2S)AuCl and the corresponding amount of the chiral
diphosphane ligand, followed by the anion interchange with
the corresponding silver salt (approx. 1 h). The resulting
suspension was filtered through a celite path and the
solution was evaporated to yield the titled complexes.10
The anion interchange was necessary because the initial
complex Binap-AuCl was inactive in terms of
enantiodiscrimination. The reactions carried out with
diisopropylethylamine (DIPEA) afforded cleaner reaction
crudes than the analogous transformations performed with
triethylamine (Table 1, entries 1 and 2). The anion
interchange on the original (Sa)-Binap-AuCl complex was
done with several silver salts (Table 1, entries 3-8) running
the cycloaddition in the presence of DIPEA. The best
Corresponding author. Tel.: +35-965903728; fax: +35-965903549; e-mail: cnajera@ua.es
2
Tetrahedron: Asymmetry
results were achieved when using the benzoate or TFA
anions (high conversions and 74% ee each, Table 1, entries
7 and 8). The chiral gold(I)-TFA complex was selected
because higher enantiodiscriminations were achieved and
the reaction products were obtained with high purity.
Another bases, such as Et3N, DABCO or DBU did not
improve the results achieved employing DIPEA as base
(Table 1, 9-11). Other solvents like THF, Et2O, and DCM
did not improve the result described in the reaction run with
toluene.
A very important feature of these carboxylate anions is the
weak basicity, which was enough to promote the identical
enantioselective cycloadditions in the absence of base
associated with an unexpected increment of the
enantioslectivity (Table 1, entries 12-18). TFA anion was
the most suitable internal base to promote this reaction in
high conversions, good enantioselectivities and affording
very clean crude reaction products. A lower catalyst
loading (5 mol%) decreased the conversion and the
enantioselection of the process (Table 1, entry 17), whilst
the formation of the gold(I) complex overnight did not
affect to the final result of the reaction (Table 1, entry 18).
O
N
O
O
Ph
(NMM)
Au complex (10 mol%)
N
PhMe, base or not
rt, 16 h
1a
Scheme 1.
CO2Me
N
O
Table 1. Optimization of the chiral gold(I)-catalyzed 1,3-DC between
iminoester 1a and NMM.
Entry
N
H
endo-2
CO2Me
Base
Conv.
(10 mol%)
(%)
(%)c
1
(Sa)-Binap-AuCl
Et3N
>95
rac.
2
(Sa)-Binap-AuCl
DIPEA
>95
rac.
3
(Sa)-Binap-AuCl/AgSbF6
DIPEA
<10
___
4
(Sa)-Binap-AuCl/AgClO4
DIPEA
>95
60
5
(Sa)-Binap-AuCl/AgOAc
DIPEA
>95
62
6
(Sa)-Binap-AuCl/AgOTf
DIPEA
>95
59
7
(Sa)-Binap-AuCl/AgOBz
DIPEA
>95
74
8
(Sa)-Binap-AuCl/AgTFA
DIPEA
>95
74
9
(Sa)-Binap-AuCl/AgTFA
Et3N
>95
92d
10
(Sa)-Binap-AuCl/AgTFA
DABCO
<50
___d
11
(Sa)-Binap-AuCl/AgTFA
DBU
<30
___d
12
(Sa)-Binap-AuCl/AgOAc
_____
>95
70
13
(Sa)-Binap-AuCl/AgOBz
_____
>95
94d
14
(Sa)-Binap-AuCl/AgTFA
_____
>95
94
(Ra)-Binap-AuCl/AgTFA
_____
>95
ent-94
(Sa)-Binap-(AuCl)2/AgTFA
_____
>95
rac.
_____
<90
60
_____
>96
94
15
17
18
e
(Sa)-Binap-AuCl/AgTFA
f
(Sa)-Binap-AuCl/AgTFA
a,b
ee
(10 mol%)
16
Ph
Gold(I) catalyst
a
Determined by 1H NMR of the crude samples.
b
The observed endo:exo ratio was always >98:2 (1H NMR)
c
Determined by chiral HPLC analysis (Daicel, Chiralpak AS).
d
Notable amounts of unidentified side products were observed (1H NMR).
e
The reaction was performed with 5 mol% of gold(I) complex.
f
The anion interchange was allowed overnight instead of 1 h.
A series of iminoesters and maleimides were tested
following the best reaction conditions described in entry 14
of Table 1 and directly compared with identical
transformations carried out with AgTFA (Scheme 2 and
Table 2). In all of the examples described in this Table 2
the endo:exo diastereoselectivity was very high (>98:2,
determined by 1H NMR spectroscopy) independently of the
central metal nature (10 mol%). In general the base-assisted
reaction was complete in 16 h giving rise to elevated
chemical yields and better enantioselections with the chiral
silver complex. However, the absence of base is much
more beneficial for the reactions run with chiral gold(I)
complex
affording
both
excellent
yields
and
enantioselections (Table 2, compare entries 1-6). It was
remarkable the result obtained when NPM was employed
as dipolarophile. A racemic product 2ac could be only
obtained with the silver(I) catalyst, whilst a 80% ee of this
cycloadduct was obtained in the gold(I)-promoted
Tetrahedron: Asymmetry
cycloaddition (Table 2, entries 5 and 6). For other
arylideneaminoesters 1b-d the behaviour was very similar
obtaining a higher enantiodiscrimination for the gold(I)catalysed processes (Table 2, entries 7-9). When substrate
1e (derived from 2-naphthalenecarbaldehyde) the
enantioselectivity of the silver(I)-catalysed process was
higher than the ee generated by the analogous reaction
developed by the (Sa)-BinapAuTFA complex (Table 2,
entry 10).
O
Ar
R1
N
N
O
(Sa)-Binap-AuTFA (10 mol%)
or (Sa)-Binap-AgTFA (10 mol%)
CO2Me
toluene, base or not
rt, 16 h
R1
N
O
Ar
1
R1 = Me (NMM), Et (NEM), Ph (NPM)
N
H
3
by (Sa)-BinapAgTFA complex justified the importance of
this complex in this cycloaddtition.
O
N
O
(Sa)-Binap-AuTFA (10 mol%)
or (Sa)-Binap-AgTFA (10 mol%)
Ph
Ph
N
Ph
N
H
O
CO2Me
toluene, base or not
rt, 16 h
N
3
O
CO2Me
Ph
endo-4
AuI complex 3 d 78%, 99% ee
AgI complex 2 d 95%, 65% ee
O
CO2Me
endo-2
Scheme 2.
The insertion of a bulky substituent at the α-position of the
1,3-dipole precursor was next evaluated. Thus, methyl
benzylideneiminophenylalaninate 3 was allowed to react
with NMM under the standard reaction conditions (Scheme
3). The reaction performed with the gold(I) complex
needed 24 h more than the corresponding reaction using the
analogous silver(I) complex for achieving almost total
conversions. The high enantioselection showed by (Sa)BinapAuTFA complex (99% ee) versus the 65% ee induced
Scheme 3.
According to our experience with the results obtained from
the application of chiral Binap-silver(I) complexes in the
enantioselective 1,3-DC of azomethine ylide and
electrophilic alkenes,5j,k,m we also tested the efficiency of
the two complexes in the enantioselective cycloaddition of
azomethine
ylides
and
trans-1,2bis(phenylsulfonyl)ethylene as synthetic equivalent of
acetylene (Scheme 4 and Table 3). The reaction performed
with gold(I) catalyst offer the best enantioselectivities of
cycloadducts 5 using a 5 mol% loading and identical
quivalents of DIPEA (Table 3, entries 2, 5 and 8). Lower
enantiomeric excesses were determined when higher
amounts of catalyst and DIPEA (10 mol%, Table 3, entries
1, 4, and 7) were used, and definitively no reaction was
observed in absence of base (Table 3, entries 3, 6, and 9).
Table 2. 1,3-DC between iminoglycinates 1 and maleimides.
(Sa)-BinapAuTFA
(Sa)-BinapAgTFA
Entry
1
Ar
R1
Base
2
Yield (%)a,b
ee (%)c
Yield (%)a,b
ee (%)c
1
1a
Ph
Me
DIPEA
2aa
quant.
70
quant.
99
2aa
90
99
quant.
99
2
1a
Ph
Me
_____d
3
1a
Ph
Et
DIPEA
2ab
quant.
70
90
99
2ab
quant.
99
91
99
4
1a
Ph
Et
_____d
5
1a
Ph
Ph
DIPEA
2ac
90
64
88
rac.
2ac
92
80
quant.
rac.
6
1a
Ph
Ph
_____d
7
1b
2-MeC6H4
Me
_____d
2ba
86
88
90
70
8
1c
2-ClC6H4
Me
_____d
2ca
88
99
92
85
Me
_____d
2da
95
>99
quant.
99
Me
_____d
2ea
94
91
quant.
99
1e
9
10
1f
4-(MeO)C6H4
2-Naphthyl
a
Isolated yields after flash chromatography (silica gel).
b
The observed endo:exo ratio was always >98:2 (1H NMR)
c
Determined by chiral HPLC analysis.
d
The reaction needed 48 h for completion.
e
The reaction was performed with 5 mol% of gold(I) complex.
f
The anion interchange was allowed overnight instead of 1 h.
4
Tetrahedron: Asymmetry
SO2Ph
PhO2S
Ar
N
(Sa)-Binap-AuTFA (5 mol%) or
(Sa)-Binap-AgTFA (5 mol%)
PhO2S
toluene, rt, 48 h
DIPEA (5 mol%)
Ar
CO2Me
1
The silver(I) catalyst operated in the absence of base and
employing a 10 mol% of caltalyst loading, and the
reduction of this amount did not produce such as significant
changes in enantioselections as gold(I) catalyst did (Table
3, compare entries 1-9). Taking in account all the possible
combinations the best enantioselections were obtained in
the presence of (Sa)-BinapAuTFA complex (5 mol%).
SO2Ph
CO2Me
N
H
endo-5
Scheme 4.
Table 3. 1,3-DC between iminoglycinates 1 and trans-1,2-bis(phenylsulfonyl)ethylene.
(Sa)-BinapAuTFA
(Sa)-BinapAgTFA
ee (%)
Yield (%)a,b
ee (%)c
80
86
quant.
92
5a
74
99
80
86
5a
_____
_____
76
96
Entry
1
Ar
Catalyst
DIPEA
5
Yield (%)
1
1a
Ph
10 mol%
10 mol%
5a
2
1a
Ph
5 mol%
5 mol%
a,b
c
2
1a
Ph
10 mol%
_____
3
1d
4-MeC6H4
10 mol%
10 mol%
5d
81
88
79
96
4
1d
4-MeC6H4
5 mol%
5 mol%
5d
78
99
79
96
5
1d
4-MeC6H4
10 mol%
_____
5d
_____
_____
74
96
6
1g
3-Pyridyl
10 mol%
10 mol%
5g
73
96
75
92
7
1g
3-Pyridyl
5 mol%
5 mol%
5g
73
99
75
98
10 mol%
_____
5g
_____
_____
78
96
8
1g
3-Pyridyl
a
Isolated yields after flash chromatography (silica gel).
b
The observed endo:exo ratio was always >98:2 (1H NMR)
c
Determined by chiral HPLC analysis.
One of the main drawbacks of the Binap-silver(I) catalysed
1,3-DC was the reaction with acrylates. In spite of using the
best reaction conditions (see above) of Table 1, the reaction
of 1,3-dipole precursors 1 with tert-butyl acrylate only
produced racemic mixtures of cycloadducts. However,
when iminoesters 6 or 7 (appropriate starting materials to
synthesize hepatitis C virus inhibitors) 11 were allowed to
react with tert-butyl acrylate catalysed by (Sa)-BinapAuTFA, at rt, for 48h, in the presence of base (Et3N, 10
mol%, rather than DIPEA) intermediates 8 or 9 were
isolated with different success (Scheme 5a, and b). Whilst
thienyl derivative 6 furnished a racemic endo-cycloadduct
8, thiazole derivative 4 generated proline endo-6 with a
relative good enantiomeric excess (Scheme 5c). The best
encouraging result was obtained when this last reaction was
performed at 0 ºC furnishing endo-cycloadduct 9 in good
isolated yield (88%) and excellent enantioselectivity (99%
ee). In both of the reported examples, the unique
diastereoisomer identified in the crude product 1H NMR
spectra was the endo-isomer.
The absolute configuration of the endo-cycloadducts was
assigned according to the chiral HPLC retention times and
by comparison of the physical properties of the isolated
samples with the properties published in the literature for
the analogous compounds.
(a)
ButO2C
N
CO2Me
ButO2C
N
H
S
S
6
(Sa)-Binap-AuTFA
(10 mol%)
(b)
toluene, rt, 48 h
Et3N (10 mol%)
N
N
CO2Me
endo-8
86%, racemic
ButO2C
N
CO2Me
S
N
H
S
7
CO2Me
endo-9
89%, 78% ee
ButO2C
(c)
ButO2C
N
N
S
7
Scheme 5.
CO2Me
(Sa)-Binap-AuTFA
(10 mol%)
toluene, 0 ºC, 48 h
Et3N (10 mol%)
N
S
N
H
CO2Me
endo-9
88%, 99% ee
Tetrahedron: Asymmetry
5
To the best of our knowledge (Sa)-Binap-(AuTFA)2, (Sa)Binap-AuTFA has not been described yet. X-Ray
diffraction analysis of did not reveal a regular pattern, such
as occurred in the X-ray diffraction analysis of the
analogous complex formed by mixing one equivalent of
(Sa)-Binap and silver perchlorate. The experience showed
that a possible mixture of linear gold aggregates (even
dimers, trimers o tetramers) can coexist in both solid state
or in solution12. This fact was observed in solution (CDCl 3)
employing 31P NMR analysis. (Sa)-Binap-AuTFA showed a
multiplet centered at 17.3 ppm, a singlet at 23.3 ppm [also
observed for the (Sa)-Binap-(AuTFA)2 complex], and a
singlet at 41.0 ppm. ESI experiments also revealed the
existence of a M+ peak at 819 corresponding to the cationic
species Binap-Au+ when the elution mixture was formed by
acetonitrile and water.
We can conclude that, in spite of the higher costs and the
employment of silver trifluoroacetate to form it, chiral (Sa)Binap-AuTFA complex presented some advantages in the
catalytic enantioselective 1,3-DC of azomethine ylides and
alkenes, versus the reaction mediated by (Sa)-BinapAgTFA complex. These two catalysts worked as
multifunctional catalysts13 in some reactions of the text,
because they were able to activate, both dipole and
dipolarophile, and also act as an inner base. The gold(I)
complex induced higher enantioselections when more
sterically hindered substrates were used, for example, NPM
and α-substituted iminoesters, which were difficult to
control by chiral silver(I) complexes. A direct consequence
of this fact is the easy access to the precursor of an antiviral
agent (99% ee) when less than 40% ee, was achieved by all
of the silver(I) tested in this and in previous works. These
particular features are currently being investigated by
computational calculations whose definitive data will be
reported in due course.
Acknowledgments
This work has been supported by the DGES of the Spanish
Ministerio de Educación y Ciencia (MEC) (Consolider
INGENIO 2010 CSD2007-00006, and CTQ200762771/BQU),
Generalitat
Valenciana
(PROMETEO/2009/039), and by the University of
Alicante. MM-R thanks MEC for a predoctoral fellowship.
References
3
1
2
Lipshutz, B. H; Yamamoto, Y. Eds. Chem. Rev. 2008, 108,
2793-3442
(a) P. Karoyan, S. Sagan, O. Lequin, J. Quancard, S. Lavielle,
G. Chassaing Targets Heterocycl. Syst. 2004, 8, 216-273; (b) C.
Nájera, J. M. Sansano, Chem. Rev. 2007, 107, 4273-4303; (c) M. I.
Calaza, C. Cativiela, Eur. J. Org. Chem. 2008, 3427-3448.
For recent reviews, see: (a) Nájera, C.; Sansano, J. M.; Yus, M. J.
Braz. Chem. Soc. 2010, in press; (b) Nájera, C.; Sansano, J. M.
Topics Heterocyclic Chem., Ed. A. Hassner 2008, 12, 117-146; (c)
Stanley, L. M.; Sibi, M. P. Chem. Rev. 2008, 108, 2887-2902; (d)
Álvarez-Corral, M.; Muñoz-Dorado, M.; Rodríguez-García, I. Chem.
Rev. 2008, 108, 3174-3198; (e) Naodovic, M.; Yamamoto, H. Chem.
Rev. 2008, 108, 3132-3148; (f) Nair, V.; Suja, T. D. Tetrahedron
2007, 63, 12247-12275; (g) Pandey, G.; Banerjee, P.; Gadre, S. R.
Chem. Rev. 2006, 106, 4484-4517; (h) Pinho e Melo, T. M. V. D.
Eur. J. Org. Chem. 2006, 2873-2888; (i) Bonin, M.; Chauveau, A.;
6
4
5
6
7
8
Tetrahedron: Asymmetry
Micouin, L. Synlett 2006, 2349-2363; (j) Nájera, C.; Sansano J. M.
Angew. Chem. 2005, 44, 6272-6276; (k) Husinec, S.; Savic, V.
Tetrahedron: Asymmetry 2005, 16, 2047-2061.
For the first enantioselective 1,3-DC catalysed by stoichiometric
amounts of Ag+ complexes, see: (a) Allway, P.; Grigg, R.
Tetrahedron Lett. 1991, 32, 5817-5820; (b) Grigg, R. Tetrahedron:
Asymmetry 1995, 6, 2475-2486.
a) Longmire, J. M.; Wang, B.; Zhang, X. J. Am. Chem. Soc. 2002,
124, 13400-13401; b) Chen, C.; Li, X.; Schreiber, S. L. J. Am. Chem.
Soc. 2003, 125, 10174-10175; c) Knöpfel, T. F.; Aschwanden, P.;
Ichikawa, T.; Watanabe, T.; Carreira, E. M. Angew. Chem. Int. Ed.
2004, 43, 5971-5973; d) Zheng, W.; Zhou, Y.-G. Org. Lett. 2005, 7,
5055-5058; e) Stohler, R.; Wahl, F.; Pfaltz, A. Synthesis 2005, 14311436; f) Zheng, W.; Zhou, Y.-G. Tetrahedron Lett. 2007, 48, 46194622; g) Zheng, W.; Chen, G.-Y.; Zhou, Y. G.; Li, Y.-X. J. Am.
Chem. Soc. 2007, 129, 750-751; h) Nájera, C.; Retamosa, M. G.;
Sansano, J. M. Org. Lett. 2007, 9, 4025-4028; i) Nájera, C.;
Retamosa, M. G.; Sansano, J. M.; de Cózar, A.; Cossío, F. P.
Tetrahedron: Asymmetry 2008, 19, 2913-2923; j) Nájera, C.;
Retamosa, M. G.; Sansano, J. M. Angew. Chem. Int. Ed. 2008, 47,
6055-6058; k) Nájera, C.; Retamosa, M. G.; Martín-Rodríguez, M.;
Sansano, J. M.; de Cózar, A.; Cossío, F. P. Eur. J. Org. Chem. 2009,
5622-5634; l) Yu, S. B.; Hu, X.-P.; Deng, J.; Wang, D.-Y.; Duan, Z.C.; Zheng, Z. Tetrahedron: Asymmetry 2009, 20, 621-625; m)
Martín-Rodríguez, M.; Nájera, C., Sansano, J. M.; Costa, P. R. R.;
Evanoel Crizanto de Lima, E.; Dias A. G. Synlett 2010 in press.
(a) Gao, W.; Zhang, X.; Raghunath, M. Org. Lett. 2005, 7, 42414244; (b) Cabrera, S.; Gómez-Arrayás, R.; Carretero, J. C. J. Am.
Chem. Soc. 2005, 127, 16394-16395; (c) Yan, X.-X.; Peng, Q.;
Zhang, Y.; Zhang, K.; Hong, W.; Hou, X.-L.; Wu, Y.-D. Angew
Chem. Int. Ed. 2006, 45, 1979-1983; (d) Llamas, T.; Gómez-Arrayás,
R.; Carretero, J. C. Org. Lett. 2006, 8, 1795-1798; (e) Llamas, T.;
Gómez-Arrayás, R.; Carretero, J. C. Synthesis 2007, 950-956; (f) Shi,
J.-W.; Shi, J. W. Tetrahedron: Asymmetry 2007, 18, 645-650; (g)
Martín-Matute, B.; Pereira, S. I.; Peña-Cabrera, E.; Adrio, J. A.;
Silva, M. S.; Carretero, J. C. Adv. Synth. Catal. 2007, 349, 17141724; (h) Cabrera, S.; Gómez-Arrayás, R.; Martín-Matute, B.;
Cossío, F. P.; Carretero, J. C. Tetrahedron 2007, 63, 6587-6602; (i)
López-Pérez, A.; Adrio, J.; Carretero, J. C. J. Am. Chem. Soc. 2008,
130, 10084-10085; (j) Fukuzawa, S.; Oki, H. Z. Y. Org. Lett. 2008,
10, 1747-1750; (k) Wang, C.-J.; Liang, G.; Xue, Z, Y.; Gao, F. J. Am.
Chem. Soc. 2008, 130, 17250-17251; (l) Kim, H. Y.; Shih, H.-J.;
Knabe, W. E.; Oh, K. Angew. Chem. Int. Ed. 2009, 48, 7420-7423;
(m) López-Pérez, A.; Adrio, J.; Carretero, J. C. Angew. Chem. Int.
Ed. 2009, 48, 340-343; (n) Hernández-Toribio, J.; Gómez-Arrayás,
R.; Martín-Matute, B.; Carretero, J. C. Org. Lett. 2009, 11, 393-396;
(o) Wang, C.-J.; Xue, Z. Y.; Liang, G.; Lu, Z. Chem. Commun. 2009,
2905-2907.
For copper(II)-catalyzed 1,3-dipolar cycloadditions see: Oderaotoshi,
Y.; Cheng, W.; Fujitomi, S.; Kasano, Y.; Minakata, S.; Komatsu, M.
Org. Lett. 2003, 5, 5043-5046.
For zinc(II) catalyzed 1,3-dipolar cyaloaditions, see: (a) Gothelf, A.
S.; Gothelf, K. V.; Hazell, R. G.; Jørgensen, K. A. Angew. Chem. Int.
Ed. 2002, 41, 4236-4238; (b) Dogan, O.; Koyuncu, H.; Garner, P.;
Bulut, A.; Youngs, W. J.; Panzner, M. Org. Lett. 2006, 8, 4687-4690.
For nickel(II) catalyzed 1,3-dipolar cycloadditions, see: Shi, J.-W.;
Zhao, M.-X.; Lei, Z.-Y.; Shi, M. J. Org. Chem. 2008, 73, 305-308.
For calcium(II) catalyzed 1,3-dipolar cycloadditions, see: (a) Saito,
S.; Tsubogo, T.; Kobayashi, S. J. Am. Chem. Soc. 2007, 129, 53645365; (b) Tsubogo, T.; Saito, S.; Seki, K.; Yamashita, Y.; Kobayashi,
S. J. Am. Chem. Soc. 2008, 130, 13321-13322.
The employment of organocatalysts has been reported: (a) Alemparte,
C.; Blay, G.; Jørgensen, K. A. Org. Lett. 2005, 7, 4569-4572; (b)
Arai, S.; Takahashi, F.; Tsuji, R.; Nishida, A. Heterocycles 2006, 67,
495-501; (c) Ibrahem, I.; Ríos, R.; Vesely, J.; Córdova, A.
Tetrahedron Lett. 2007, 48, 6252-6257; (d) Xue, M.-X.; Zhang, X.M.; Gong, L.-Z. Synlett 2008, 691-694; (e) Vicario, J. L.; Reboredo,
S.; Badía, D.; Carrillo L. Angew. Chem. Int. Ed. 2007, 46, 5168-5170;
(f) Agbodjan, A. A.; Cooley, B. E.; Copley, R. C. B.; Corfield, J. A.;
Flanagan, R. C.; Glover, B. N.; Guidetti, R.; Haigh, D.; Howes, P. D.;
Jackson, M. M.; Matsuoka, R. T.; Medhurst, K. J.; Millar, A.; Sharp,
M. J.; Slater, M. J.; Toczko, J. F.; Xie, S. J. Org. Chem. 2008, 73,
3094-3087; (g) Flanagan, R. C.; Xie, S.; Millar, A. Org. Process Res.
Develop. 2008, 12, 1307-1312; (h) Nakano, M.; Terada, M. Synlett
2009, 1670-1674; (i) Yu, L, He, L.; Chen, X.-H.; Song, J.; Chen, W.J. Gong, L.-Z. Org. Lett. 2009, 11, 4946-4949; (j) Chen, X.-H.;
Zhang, W.-Q.; Gong, L.-Z. J. Am. Chem. Soc. 2009, 130, 5652-5654;
(k) Chen, X.-H.; Wei, Q.; Luo, S.-W.; Xiao, H.; Gong, L.-Z. J. Am.
Chem. Soc. 2009, 130, 13819-13825.
9 Widenhoefer, R. A. Chem. Eur. J. 2008, 14, 5382-5381.
10 Melhado, A. D.; Luparia, M.; Toste, F. D. J. Am. Chem. Soc.
2007, 129, 12638-12639.
11 Nájera, C.; Sansano J. M. Org. Biomol. Chem. 2009, 7, 45674581.
12 Wheaton, C. A.; Jennings, M. C.; Puddephatt, R. J. J. Am.
Chem. Soc. 2006, 128, 15370-15371.
13 For reviews about multifunctional catalysis see: (a) Shibasaki,
M.; Kanai, M.; Matsunaga, S. Aldrichimica Acta 2006, 39,
31-39; (b) Kanai, M.; Kato, N.; Ichikawa, E.; Shibasaki, M.
Pure Appl. Chem. 2005, 77, 2047-2052; (c) Kanai, M.; Kato,
N.; Ichikawa, E.; Shibasaki, M. Synlett 2005, 1491-1508; (d)
Ma, J.-M.; Cahard, D. Angew. Chem., Int. Ed. 2004, 43, 45664583; (e) Shibasaki, M.; Kanai, M.; Funabashi, K. Chem.
Commun. 2002, 1989-1199; (f) Matsunaga, S.; Ohshima, T.;
Shibasaki, M. Adv. Synth. Catal. 2002, 344, 3-15; (g) Gröger,
H. Chem. Eur. J. 2001, 7, 5246-5251; (h) Rowlands, G. J.
Tetrahedron 2001, 57, 1865-1882.






