NORMALITY
advertisement
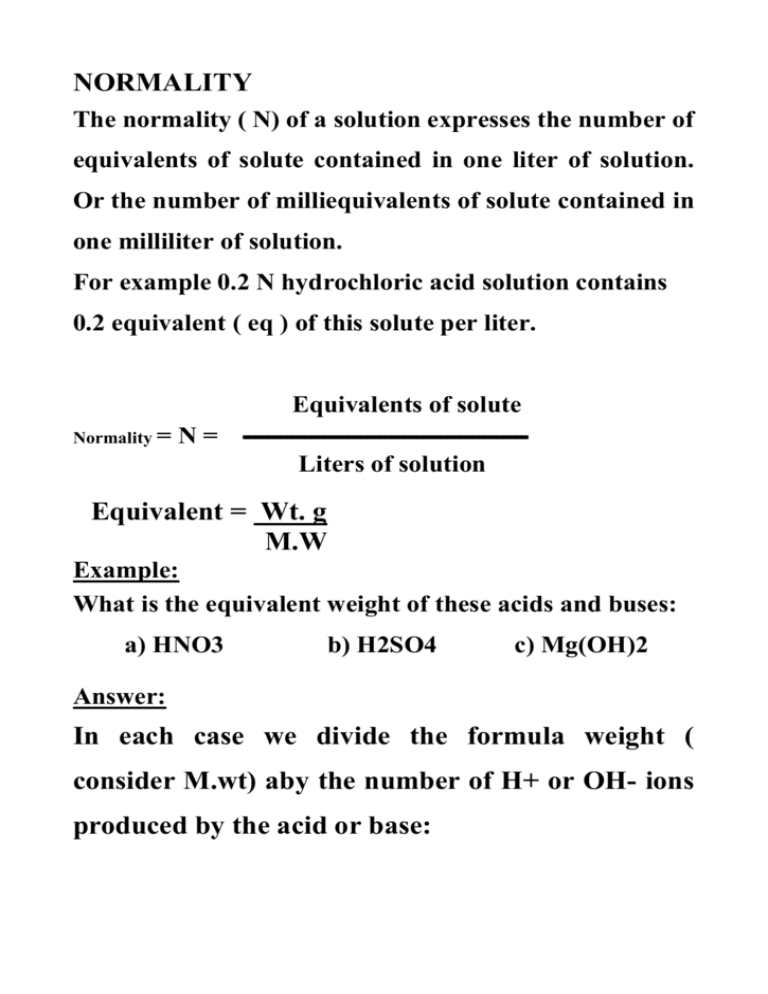
NORMALITY The normality ( N) of a solution expresses the number of equivalents of solute contained in one liter of solution. Or the number of milliequivalents of solute contained in one milliliter of solution. For example 0.2 N hydrochloric acid solution contains 0.2 equivalent ( eq ) of this solute per liter. Equivalents of solute Normality = N= ــــــــــــــــــــــــــــــــــــــــــــــ Liters of solution Equivalent = Wt. g M.W Example: What is the equivalent weight of these acids and buses: a) HNO3 b) H2SO4 c) Mg(OH)2 Answer: In each case we divide the formula weight ( consider M.wt) aby the number of H+ or OH- ions produced by the acid or base: 63 a) HNO3 : Eq.wt. = 63 = 1 b) H2SO4 : Wq.wt. = 98 = 49 2 c) Mg ( OH)2 : Eq.wt. = 58 2 = 29 Example 2 : What is the normality of a solution made by dissolving 8.5g of H2SO4 in inough water to malce 500ml of solution ? Answer: First, we need the number of equivalents of H2SO4 per liter of solution, and we know (from the above example) that the equivalent weight of H2SO4 is 49. Thus: Equivalent of H2 SO4 = weight ( g) = 8.5 = 0.17 Eg . wt 49 To find normality N we divide equivalents pf H2SO4 by liters of solution: (Note: 500ml = 0.5L) N= 0.17 0.5 = 0.34 N. ** The relationship between Normality and Molarity: Where n = the number of replaceable H+ or OHper molecule ( for acids and bases ) Or n the number of electrons lost or gained per molecule ( for oxidizing and reducing agents ). The molarity and normality are related by : N = nM Example: What is the normality of the 0.01M solution of H2SO4? Answer: N = nM in H2SO4 n = 2 : N = 2 x 0.01 = 0.02 N Answer 1:a) M = mol liter mole = 0.04 = mol 0.5 0.02 = g . mwt moles = 0.5 x 0.04 = 0.02 g 40 g = 40 x 0.02 = 0.8 g We need 0.8 g to dissolve it in water and dilute to 500 ml to get the desired conc. 0.04M NaOH. b) * NaOH contains one OH per molecule, thus : M = N cind the solution is 0.04N * The solution contains 0.8g in the 500 ml 1.6 g /liter * %(w/v) = g per 100 ml 1.6 g/ 100 ml liter = 1.6/ 1000 ml = 0.16 g / 0.16 % * mg % = mg per 100ml 0.16 g /100 160 mg / 100 ml = 160 mg % Example 2 : - How many ml of 5M H2 SO4 are required to made 1500 ml of a 0.002 M H2 SO4 solution ? Answer 2:- C x V (for conc. ) = C x V (for dilute) 5 M x V = 0.002M x 1500 ml V = 0.002 × 1500 = 0.6 ml 5 We need 0.6 ml of the conc. Solution and dilute it to 1.5 Kuters.











