Cysteine proteinase pathway required for Drosophila long
advertisement
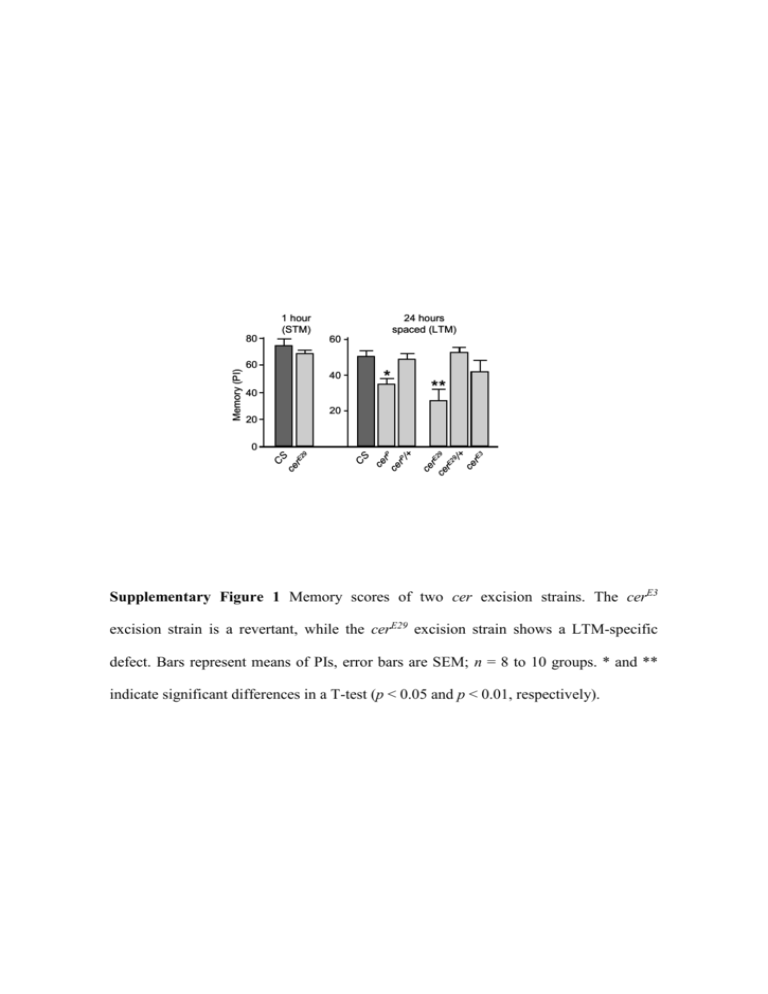
Memory (PI) 80 1 hour (STM) 24 hours spaced (LTM) 60 60 40 * 40 ** 20 20 3 ce rE ce 29 r E2 9 /+ ce rE CS ce rP ce rP /+ 9 r E2 ce CS 0 Supplementary Figure 1 Memory scores of two cer excision strains. The cerE3 excision strain is a revertant, while the cerE29 excision strain shows a LTM-specific defect. Bars represent means of PIs, error bars are SEM; n = 8 to 10 groups. * and ** indicate significant differences in a T-test (p < 0.05 and p < 0.01, respectively). a ... ... ... ... ... ... ... ... ... ... b Cathepsin L 1000 1/V 800 x 600 x x 400 x x x x 6 μM Subs. 4.5 μM Subs. 3 μM Subs. -20 0 20 40 60 80 100 [Cer] (nM) Supplementary Figure 2 Cer is a cysteine proteinase inhibitor. a, BLAST alignment results are presented. “…” indicates incomplete sequence. Cer is highly similar to cysteine proteinase inhibitors such as murine Cytotoxic T-Lymphocyte Antigen-2 alpha and beta (CTLA-2 and CTLA-2)1 and Bombyx mori Cysteine proteinase inhibitor (BCPI)2. Some cysteine proteinases are also shown: Drosophila Cysteine proteinase 1 (CP1) and B. mori cysteine proteinase (BMCP). The degree of similarity is 44% between Cer and CTLA-2 and 58% between Cer and CP1 if one only takes into account the CP1 region that aligns with Cer. Drosophila CP1 is a member of the cathepsin L subfamily3. Sequence analysis suggests that cer arose by partial duplication of the first three exons of an ancestral CP1 gene4. To our knowledge, no cer mutant has been previously isolated. Unlike BCPI and CTLA-2, Cer lacks an N-terminal hydrophobic leader sequence, suggesting that it is a cytoplasmic protein, while BCPI and CTLA-2 are likely granular or secretory proteins4. b, Cer can inhibit human cathepsins L. Dixon plot of Cer inhibition of cathepsin L. The Dixon plot is a convenient way of calculating the Ki of an inhibitor. The reaction velocity is measured at a fixed concentration of substrate, but at a variety of inhibitor concentrations. A graph of the reciprocal of velocity against inhibitor concentration is plotted. This is repeated at different substrate concentrations and a similar line drawn for each concentration of substrate. A vertical line dropped from the point where the lines intersect each other down to the inhibitor axis gives -Ki. Calculated Ki = 25 nM. Cysteine proteinase inhibitors can inhibit different types of cathepsins, due to their three dimensional structure5. BCPI can inhibit cathepsins L and H but not cathepsin B or papain6. On the other hand, CTLA-2 inhibits cathepsin L and papain but not cathepsin B7. Cer inhibits cathepsin L and also cathepsin B (data not shown) but not papain (data not shown). Supplementary Figure 3 Confocal microscopy image of a 5122-Gal-4/P(UAS-mCD8GFP) brain. The 5122 line drives the membrane Green Fluorescent Protein into three different MB lobes: / (yellow) and (red). The scale bar represents 40 m. Supplementary reference list: 1. Denizot, F. et al. Novel structures CTLA-2 alpha and CTLA-2 beta expressed in mouse activated T cells and mast cells and homologous to cysteine proteinase proregions. Eur J Immunol 19, 631-5 (1989). 2. Yamamoto, N., Hegde, A. N., Chain, D. G. & Schwartz, J. H. Activation and degradation of the transcription factor C/EBP during long-term facilitation in Aplysia. J Neurochem 73, 2415-23 (1999). 3. Matsumoto, I., Watanabe, H., Abe, K., Arai, S. & Emori, Y. A putative digestive cysteine proteinase from Drosophila melanogaster is predominantly expressed in the embryonic and larval midgut. Eur J Biochem 227, 582-7 (1995). 4. Yamamoto, Y., Kurata, M., Watabe, S., Murakami, R. & Takahashi, S. Y. Novel cysteine proteinase inhibitors homologous to the proregions of cysteine proteinases. Curr Protein Pept Sci 3, 231-8 (2002). 5. Turk, D., Podobnik, M., Kuhelj, R., Dolinar, M. & Turk, V. Crystal structures of human procathepsin B at 3.2 and 3.3 Angstroms resolution reveal an interaction motif between a papain-like cysteine protease and its propeptide. FEBS Lett 384, 211-4 (1996). 6. Kurata, M. et al. Bombyx cysteine proteinase inhibitor (BCPI) homologous to propeptide regions of cysteine proteinases is a strong, selective inhibitor of cathepsin L-like cysteine proteinases. J Biochem (Tokyo) 130, 857-63 (2001). 7. Delaria, K. et al. Inhibition of cathepsin L-like cysteine proteases by cytotoxic Tlymphocyte antigen-2 beta. J Biol Chem 269, 25172-7 (1994).