Supplementary Text 3: Stability and sensitivity analysis
advertisement

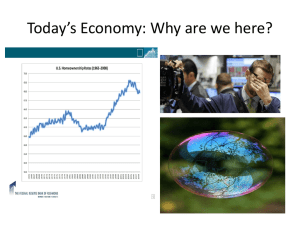
Supplementary Text 3: Stability and sensitivity analysis The stability and sensitivity analysis of the GMA model for total masses (Supplementary Text 5) was executed with the software PLAS4, which uses a numerical gradient method to determine sensitivities. The system is asymptotically stable at the baseline steady state, as well as other states tested, as indicated by the fact that all the eigenvalues have negative real parts; four imaginary components suggest the potential for oscillations in the system. The robustness of a model is assessed by means of parameter sensitivities, which are easily computed for S-system and GMA systems1,2. It is usually beneficial for a model to have sensitivities of small magnitude3, with values less than 1 indicating attenuation of a perturbation and values above 1 predicting amplification. The sign of a sensitivity value furthermore describes the direction of the change in output variable. Overall, a robust model exhibits a sensitivity profile with mostly small values. The results are shown in Supplementary Table 3. This represents the general analysis for magnitude sensitivities. Most of the kinetic order sensitivities with respect to metabolites (64.8 %) and fluxes (71.5 %) are less than one. Similarly, most of the rate constant sensitivities are less than one in magnitude (77.9% for metabolites and 82.4% for fluxes). These results indicate that a perturbation in a parameter of the model will be attenuated in most parts of the system. Supplementary Table 3: Sensitivities of metabolites and fluxes % of metabolites Sensitivities Logarithmic Gains Rate constants Kinetics Orders Total 975 2575 7175 <1 64.8 77.9 71.7 <2 72.7 83.2 76.4 <5 81.9 88 82.5 % of fluxes < 10 87.1 90.7 86.9 Total 3783 9991 27839 <1 71.5 82.4 74.5 <2 78.6 86.5 79.2 <5 86.3 90 85.8 < 10 92.3 94 90 Supplementary Table 3 also presents logarithmic gains, which quantify the influence exerted by independent variables on the values of the dependent variables at steady state. The magnitudes of logarithmic gains are mostly below 1 (64.8% for metabolites and 71.5% for fluxes), which indicates that most perturbations in independent variables (and thus in enzyme-catalyzed steps) will be attenuated by the system. With respect to the rate constants, about 80% of metabolites and fluxes are less than one, indicating a general attenuation of perturbations in the flux rates of the model, which could be due to changes in the specific activity of an enzyme. Detailed Analysis of Logarithmic Gains It is interesting to diagnose the results of the global robustness analysis further. Of particular importance are the logarithmic gains, which indicate how an alteration in an independent variable (enzyme activity) affects the steady state (concentrations and fluxes) of the system. As presented in Supplementary Table 3, about two thirds of the gains with respect to steady-state metabolite concentrations are below 1 in magnitude, signifying attenuation of perturbations, and about 20% of the gains have magnitudes between 1 and 10, indicating moderate amplification. The remaining 10% indicate strong amplification. Two causes may be responsible for these high gains. First, they may reflect reality, or secondly, the model may be inaccurate. Before we distinguish between these causes further, it is important to note that the high gains are certainly not randomly distributed throughout the system. In fact, studying the gain profile in Supplementary Fig. 3, one notices immediately that they are crisply clustered in identifiable portions of the system. A 250 200 150 100 50 0 26 28 30 31 32 33 34 36 37 38 X 39 40 j 43 44 46 47 48 49 51 53 54 56 57 58 60 61 62 63 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 25 24 23 22 21 20 19 18 17 X Supplementary Figure 3: Influence of the independent variables Xj on metabolite concentrations Xi, as quantified by the absolute values of the corresponding logarithmic gains L(Xi, Xj). Independent variables with logarithmic gains close to 0 are omitted. i The first cluster of high-gain dependent variables consists of dihydro-ceramide (X3), PHS (X5), PHS-P (X6) and IPC-g (X8), which show moderately strong responses to changes in a number of enzyme activities. While low gains are typical for robust metabolic pathways, signaling pathways should be expected to exhibit some high gains, lest the system would not be able to amplify an input signal with appreciable efficacy. Indeed, hormonal and other signal transduction systems, such as the MAP kinase network, sometimes have gains at orders in excess of 100. In the given case, dihydroceramide (X3), PHS (X5), PHS-P (X6), IPC-g (X8), and the very long chain fatty acids (C26-CoA; X23) have relatively high gains with respect to a number of independent variables, which suggests a responsiveness of the sphingolipid pathway that requires deeper analysis. While these gains are probably not surprising, by far the highest-gain clusters involve Acetyl-CoA (X25) with gains exceeding 200, and, somewhat less dramatic, CDP-E (X17). Acetyl-CoA is a very prevalent metabolite that is involved in numerous pathways. However, in our model it appears only once as part of the model input, and its dynamics is controlled entirely by merely a handful of variables, which in the global picture of an entire cell are probably of minor importance. This limited representation of pathways toward and from Acetyl-CoA leaves no room for effectively satisfying sudden demands from other sources or alleviating spurious accumulation. In other words, it is clear that Acetyl-CoA is not modeled well, and the analogous is true for CDP- Ethanolamine. This begs the question of whether the failure of the model to capture the dynamics of these two metabolites well compromises the overall quality of the model. The answer is No, though caution is necessary. Clearly, the model is not designed to describe the dynamics of Acetyl-CoA, and one must therefore be careful with simulations that require, or lead to, large variations in this variable. Also, responses in this variable that are observed as consequences of some perturbation are prone to misinterpretation. On the other hand, the model is designed to capture the dynamics of sphingolipids, and as long as Acetyl-CoA and CDP-E remain relatively close to their baseline levels, the observed responses in other variables are not unreasonably affected by the high gains associated with Acetyl-CoA or CDP-E. The results in Supplementary Fig. 3 are also interesting if one does not focus on the dependent variables, as we did above, but on the independent variables that exert the strongest effects. In this case, the clusters involve primarily enzymatic and transport steps at the periphery of the system. Some of these are involved in the metabolites not modeled well, especially Acetyl-CoA. Examples are G3P acyltransferase (X49), the Acyl-CoA binding protein (ACBP; X48), Acetyl-CoA synthetase (ACSp; X63), and the substrates CoA (X61) and acetate (X62). Other high-gain independent variables are related to inputs to the system. Examples in this case are palmitoyl transport and palmitoyl-CoA synthase (X30), phosphoserine-phosphatase (X31) and the serine hydroxymethyl transferase (SMT; X32). The effects of changes in these inputs are strong in some of the sphingolipids, a result that seems reasonable, and they are very pronounced in the variables not modeled well, such as Acetyl-CoA, for which these inputs are the sole source of material. Ignoring logarithmic gains that involve peripheral variables, one notices that the sphingolipids themselves are most affected by inputs to the system and secondly by enzymes that are known to be very influential, namely palmitoyl transport and palmitoyl-CoA synthase (X30), serine palmitoyltransferase (SPT; X57), and phosphatidylserine decarboxylase (X56). This result supports our confidence in the model. In summary, the majority of gains are unremarkably small in magnitude, attesting to a robust model. The high gains are not at all randomly distributed throughout the pathway but show very distinct patterns that allow specific diagnostics. Some of these higher gains are presumably real, demonstrating that the sphingolipid signaling pathway has the capacity to amplify specific inputs. Other high gains are modeling artifacts, which are unavoidable, lest the model becomes unwieldy. These artifacts are easily detected as well as interpreted. They must be taken into consideration when simulations involve metabolites that are not central to the model and therefore are not modeled with accuracy. Analyses of flux gains and of kinetic order and rate constant sensitivities yield analogous results, which are not detailed here. References 1. 2. 3. 4. Sorribas, A. & Savageau, M. A. Strategies for representing metabolic pathways within biochemical systems theory: reversible pathways. Math Biosci 94, 239-69 (1989). Voit, E. O. Computational Analysis of Biochemical Systems. A Practical Guide for Biochemists and Molecular Biologists. (Cambridge University Press, Cambridge, UK, 2000). Shiraishi, F. & Savageau, M. A. The tricarboxylic acid cycle in Dictyostelium discoideum. II. Evaluation of model consistency and robustness. J Biol Chem 267, 22919-25 (1992). Ferreira, A. E. N. (1995-2003) PLAS: http://correio.cc.fc.ul.pt/~aenf/plas.html.