SYLLABUS COURSE TITLE Cell biology Faculty/Institute Institute of
advertisement

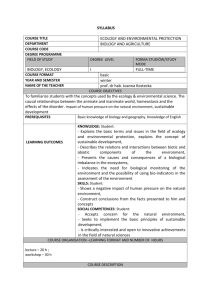
SYLLABUS CELL BIOLOGY INSTITUTE OF APPLIED BIOTECHNOLOGY AND BASIC SCIENCES COURSE TITLE FACULTY/INSTITUTE COURSE CODE DEGREE PROGRAMME FIELD OF STUDY DEGREE LEVEL FORMA STUDIÓW/STUDY MODE BS LABORATORY SECOND YEAR, SUMMER SEMESTER WNUK MACIEJ COURSE OBJECTIVES The successful student in this course will develop a deeper understanding of what life is and how it functions at the cellular level. Students will develop skills in interpreting scientific data, designing and executing cellular experiments, and communicating scientific data and concepts clearly and effectively. develop a basic knowledge and understanding of the major integrating concepts of the biological sciences (chemical basis for life, cell theory, inheritance, anatomy and physiology); develop knowledge of the structure and function of the cell. COURSE FORMAT YEAR AND SEMESTER NAME OF THE TEACHER PREREQUISITES LEARNING OUTCOMES Students are expected to possess a basic proficiency in general chemistry, organic chemistry, and biology. It is the student’s responsibility to review background materials as necessary KNOWLEDGE: Understand and utilize the scientific vocabulary used in communicating information in cell and molecular biology • Understand and apply general concepts of cell and molecular biology to relevant, specific problems • Describe and discuss the properties and biological significance of the major classes of molecules found in living organisms and the relationship between molecular structure and biological function • Represent and illustrate the structural organization of genes and the control of gene expression • Conceptualize and describe protein structure, folding and sorting • Explain the structure of membranes and intracellular compartments and relate these to function. • Summarize the processes of energy transduction in cells • Relate how cell movement and cell-cell communication occur and discuss mechanisms of signal transduction • Outline the processes that control eukaryotic cell cycle and cell death. • Link the rapid advances in cell and molecular biology to a better understanding of diseases, including cancer. SKILLS: Basic skills in pipetting, liquid handling, balances, pH meter, preparation of buffers, Sterile technique for cell culture, Cell culture and cryostorage, Isolation of plasmid DNA from E. coli, Monitoring enzymatic digestion of yeast cell wall, Cell transformation by introduction of plasmid DNA, Light and fluorescence microscopy: Detection of GFP-fusion proteins in cells Cell fractionation: cell disruption and organelle separationPreparation of soluble cell extracts and detergentsolubilized cell extracts, Affinity purification of proteins from cell extracts, Detection of protein -protein interactions, Determination of protein concentra tion by Bradford assay, Column chromatography -desalting columns, SDS PAGE analysis of proteins Western blot detection of proteins COURSE ORGANISATION –LEARNING FORMAT AND NUMBER OF HOURS 15 HRS LECTURE, 30 HRS - LABORATAORY COURSE DESCRIPTION LECTURE – 15 hrs MOLECULAR GENETICS, GENES, AND CHROMOSOMES: Nucleic Acid Structure, Transcription, and Decoding, Chromosomal Organization of Eukaryotic Genes, Chromatin Structure -3hrs CONTROL OF GENE EXPRESSION: Eukaryotic and Prokaryotic Transcription Control Elements and RNA Polymerases Cytoplasmic Posttranscriptional Control; RNA Interference; mRNA Degradation – 3hrs CELLULAR FUNCTIONS: Membranes and Membrane Transport, Protein Targeting and Transport, Cellular Energetics, Secretion and Endocytosis, Cell Signaling 3 hrs CYTOSKELETON: Cytoskeleton: Actin and Myosin, Microtubules, Intermediate Filaments, Cell Adhesion, & Extracellular Matrix 2 hrs CELL GROWTH: Cell Cycle, Stem Cells and Cell Differentiation, Regulation of Cell Death and Cancer, Cancer: Tumor Cells, Genetic Basis, Senesence and Aging 4hrs LABORATORY -30Hrs In vitro cell culture: Cell morphology, general procedure of cell culture- 3 hrs Preparation of Metaphase Spread from in vitro cell culture- 3hrs Detection centromeric sequences by Fluorescence in situ Hybridization – 6 hrs Apoptosis – Cell death tests: TUNEL assay, AO/ETBR, cell cultures for in vitro assays – 6 hrs Cell Cycle – p53 and p21 detection by immunofluorescence – 6 hrs Fluorimetric ROS assay, In vitro Thiol content determination – 3 hrs Cell Senescence – morphology and beta gal test- 3hrs. POWERPOINT PRESENTATION (LECTURE), Practical Course (Laboratory) AND Lecture –Oral presentation from Lecture subject (presentation should be limited to 60 minutes)- examination Laboratory- on the basis Research paper analysis and Written reports After the completion of an experiment, each student should prepare a journal style article for the lab report.This should include: •Abstract: concise summary of what happened during the METHODS OF INSTRUCTION REQUIREMENTS ASSESSMENTS GRADING SYSTEM TOTAL STUDENT WORKLOAD NEEDED TO ACHIEVE EXPECTED LEARNING OUTCOMES EXPRESSED IN TIME AND ECTS CREDIT POINTS LANGUAGE OF INSTRUCTION INTERNSHIP MATERIALS experiment (2-3 sentences for each experiment) •Introduction: to provide adequate b ackground pertaining to the cell line and experiments to give anyreader knowledge of why you did the experiment. This should include your hypothesis. •Materials and Methods: concise summary of what you did including how the cells were prepared and maintained. •Results: This section will include both figures and written results. Here you state simply what you saw. •Discussion: This section you will analyze your results and state why you observed what you did during the experiment. (Conclusions section) •References: you will need to be looking for references to support your introduction and discussion. 2-5 110 HRS/4 ECTS ENGLISH PRIMARY OR REQUIRED BOOKS/READINGS: Molecular biology of the cell, Fifth edition. (2008) Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter. Garland Science Publishers, New York, NY. ISBN: 978-0-8153-4105-5. SUPPLEMENTAL OR OPTIONAL BOOKS/READINGS: PUBMED- DIFFERENT SCIENTIFIC PAPERS LABORATORY AND SAFETY CONDUCT: FAILURE TO COMPLY WITH SAFETY CONDUCT WILL RESULT IN IMMEDIATE EXPULSION FROM LAB FOR THE DAY AND A ZERO WILL BE GIVEN FOR THE DAY’S ASSIGNMENTS. 1.FOOD AND DRINKS ARE NOT ALLOWED IN THE LAB 2.CLOSED TOED SHOES THAT COMPLETELY COVER THE FOOT MUST BE WORN AT ALL TIMES IN LAB. (BALLET FLATS OR SIMILAR SHOES THE EXPOSE THE TOP OF THE FOOT ARE NOT ALLOWED) 3.LONG JEANS OR PANTS MUST BE WORN. SHORTS AND CAPRIS ARE NOT ALLOWED. 4.LONG HAIR MUST BE TIED BACK AND KEPT AWAY FROM FLAME 5.LATEX OR VINYL GLOVES MUST BE WORN WHILE CONDUCTING EXPERIMENTS OR HANDLING REAGENTS ORORGANISMS 6.SPILLS OR BROKEN GLASS MUST BE REPORTED IMMEDIATELY 7.BIOLOGICAL WASTES MUST BE DISPOSED OF CORRECTLY. (MICROPIPETTE TIPS IN THE RED BOXES, PIPETTE TIPS IN THE CREAM CYLINDERS, CULTURE DISHES, TEST TUBES, ETC. IN THE BIOLOGICAL WASTE/GLASS CONTAINER BOXES) 8.AVOID BREATHING IN OR TASTING CHEMICALS–NEVER PIPETTE BY MOUTH 9.NO UNAUTHORIZED EXPERIMENTS ARE ALLOWED 10.CULTURE TUBES ARE TO BE PLACED IN A RACK, NEVER ON THE COUNTER 11.CELL PHONES SHOULD BE PLACED ON SILENT OR BE TURNED OFF 12.DISRUPTIVE BEHAVIOR WILL RESULT IN EXPULSION FROM LAB 13.REMEMBER TO USE CAUTION WHEN HANDING GLASSWARE OR SHARP TOOLS. 14.AT THE START OF EACH LAB THE BENCH TOP MUST BE WIPED OFF WITH DISINFECTANT.