View/Open - DukeSpace
advertisement

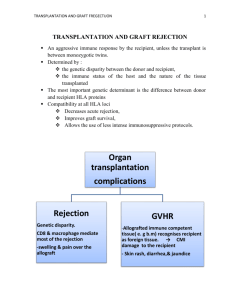
Prevention trumps treatment of antibody-mediated transplant rejection Stuart J. Knechtle, Jean Kwun, and Neal Iwakoshi Emory University School of Medicine, Atlanta, Georgia, USA. Conflict of interest: SJK acknowledges stock ownership in Bristol Myers Squibb (AUTHOR: Please review the JCI Conflict of Interest Policy for Authors outlined below and confirm or revise your COI statement as necessary) Potential conflicts to be disclosed by authors (www.jci.org/kiosk/coi): * (1) Ownership * (2) Income. If an author has received $10,000 or more of income per annum from any single private or public company in the health care field in the calendar year preceding the date of the original submission, a potential conflict must be declared. This includes any and all sources of financial benefit, including, but not limited to, consultancy, speaking fees, royalties, licensing fees, retainers, salary (including deferred compensation), honoraria, service on advisory boards, and providing testimony as an expert witness. Income generated by immediate family members (spouse or children) of the author are included. * (3) Research support. If an author’s research was funded by $50,000 or more per annum from a private or public company in the health care field in the fiscal year preceding the date of the original submission, including funding for personnel working within the laboratory, a potential conflict must be declared. Abstract 1 Belying the spectacular success of solid organ transplantation and improvements in immunosuppressive therapy is the reality that long-term graft survival rates remain relatively unchanged, in large part due to chronic and insidious alloantibody-mediated graft injury. Half of heart transplant recipients develop chronic rejection within 10 years – a daunting statistic, particularly for young patients expecting to achieve longevity by enduring the rigors of a transplant. The current immunosuppressive pharmacopeia is relatively ineffective in preventing late alloantibody-associated chronic rejection. In this issue of the Journal, Kelishadi et al. report that pre-emptive deletion of B cells prior to heart transplantation in cynomolgus monkeys, in addition to conventional post-transplant immunosuppressive therapy with cyclosporine, markedly attenuated not only acute rejection but also alloantibody elaboration and chronic graft rejection in treated animals (1). The success of this preemptive strike implies a central role for B cells in graft rejection, and this approach may help to delay or prevent chronic rejection after solid organ transplantation. Acute and Chronic Rejection Newly transplanted organs are susceptible within a week to acute rejection, mediated dominantly by T lymphocytes, but are usually effectively protected from this form of inflammation and injury by currently used immunosuppressive agents such as calcineurin-inhibitors, anti-proliferative agents, mTOR inhibitors, and prophylactic T cell antibody therapy. When acute rejection occurs, as it does in 5-25% human of solid organ recipients within the first year, such episodes typically respond to steroid therapy or, if needed, T cell antibodies. However, the current immunosuppressive pharmacopeia is relatively ineffective in preventing or treating rejection mediated by B lymphocytes and their antibody effector arm. Antibody injury typically manifests later, more insidiously, and is characterized by complement deposition and microvascular obliteration leading to tissue ischemia and eventually fibrosis with loss of function. Chronic rejection refers to such immunologically mediated allograft injury. While factors contributing to chronic injury of organ transplants are multiple and include ischemia/reperfusion injury, pre-existing donor disease, drug toxicities, and 2 recurrence of original disease, the subtle development in the graft recipient of antibodies against the foreign donor tissue (alloantibodies) in the months and years following organ transplantation has been shown to be an accurate predictor of graft failure (2, 3). The magnitude of this problem is compounded by the practical difficulties in designing feasible clinical trials to evaluate methods for preventing alloantibody development, and by the paucity of proven strategies to prevent alloantibody development in large animal models or humans. Nevertheless, data suggest that if preexisting antibody can be reduced, the risk of graft loss is lower (4). B Cell Depletion as Treatment of Established Antibody-Mediated Rejection In the medical literature, organ transplant patients experiencing antibodymediated rejection have been treated with rituximab (an anti-CD20 monoclonal antibody that depletes the B cell population) or by targeting of their plasma cells, and in most cases these patients possessed pre-existing alloantibody or suffered from early antibodymediated rejection ( 5, 6). As expected, it is difficult to reverse the damage done by alloantibody in the setting of an established B cell immune response, and the efficacy of targeting B cells with rituximab under these post-transplant circumstances has been difficult to clearly establish. The combination of B cell depletion with profound T cell immunosuppression may also be complicated by loss of protective immunity (7). In other words, infection or malignancy may ensue, especially when both T and B cell– depleting antibodies are administered simultaneously or sequentially. Therefore, an alternative strategy, that being prevention as opposed to treatment of the B cell alloimmune response, even if resorting to B cell depletion, may be attractive. Pre-emptive B Cell Depletion In their study in this issue of the Journal, Kelishadi et al. (1) show that preemptive treatment of cynomologous monkey allogeneic heart transplant recipients with rituximab on the day of the transplant substantially eliminated the injury attributable to B lymphocytes: infiltration of the graft by B cells, B cell activating factor (BAFF), B cell costimulatory molecules CD80 and 86. In addition, the downstream effects of B cell activation were attenuated including reduced alloantibody in blood and less complement 3 deposition in the graft. Perhaps most importantly, these mechanistic changes were reflected by a significant improvement in the microvascular integrity of the transplanted hearts (less chronic allograft vasculopathy) and by improved function with four of four hearts beating well by 90 days compared to only three of seven treated with cyclosporine alone. As seen from the control animals treated with cyclosporine alone, calcineurininhibitors alone were unable to effectively prevent the injury inflicted by B cell mediated rejection. Implications for Human Transplant Patients Therapeutic targeting of CD20 in transplantation may be appealing because of CD20’s stable expression primarily on B cells in the peripheral blood and its absence from plasma cells, pro-B cells, or hematopoietic stem cells, thus permitting maintenance of serum IgG levels and post-treatment recovery by spared pro-B and stem cells (8). For the same reasons, therapeutic targeting of CD20 may not be as effective in treating recipients known to have donor-specific antibody prior to transplantation since memory B cells and plasma cells capable of producing antibody to the donor organ would already be primed. Since many T lymphocyte–mediated immune responses include a B lymphocyte component, the impact of B cell depletion may extend beyond suppression of measurable antibody (9) as is suggested by the observation in the current study that acute rejection was reduced from a 57% incidence with cyclosporine alone to zero by addition of rituximab in experimental animals. A non-human primate (NHP) model is far closer, genetically, to the human condition than any rodent model might be, and thus the current report would be expected to predict better than any rodent model of transplantation how humans might respond to B cell depletion. Nevertheless, it is worth noting that even observations in NHPs in the field of organ transplantation have sometimes been difficult to translate directly into the clinic (10, 11). By analogy, human cardiac transplant patients usually receive three or four simultaneous immunosuppressive agents to prevent T cell–mediated rejection, compared to the monkeys in the current study by Kelishadi et al., which received high dose cyclosporine as their sole immunosuppressive agent (1). The applicability of the findings of the current study to human organ transplantation will require rigorous testing 4 in order to determine if pre-emptive CD20 monoclonal antibody treatment in the setting of more intense T cell immunosuppression will not be attended by opportunistic infection. Other B Cell Strategies for Transplantation Targeting B cell immunity without depleting these cells in order to prevent alloantibody development may also lead to opportunities to prevent graft injury (Figure 1). Such strategies include targeting complement pathway components (12) and B cell cytokines/chemokines such as BAFF/APRIL (B cell–activating factor belonging to the TNF family / a proliferation-inducing ligand), which may influence both B and T cell responses (13, 14). Other biologics being considered for development for the targeting of B cell responses in transplantation are those that affect the costimulatory pathways. Interactions between CD28 on CD4+ T cells and CD80/CD86 on B cells, as well as between CD28 on activated CD4+ T cells and CD40 ligand (CD40L, also known as CD154) on B cells have been shown to participate in providing T cell help to B cells (15) The CD40/CD40L interaction stimulates B cell proliferation and isotype switching in the appropriate cytokine milieu (16, 17). CD28/CTLA-4 (cytotoxic T-lymphocyte antigen 4) expression has also been shown to be involved in germinal center formation (18). Each of these potential therapies is under active investigation. The relative safety and efficacy of such strategies compared to profound B cell depletion with rituxumab will be important to compare. Additionally, it will be necessary to determine the durability and need for repeated application of B cell therapy in the setting of constant exposure to alloantigen, as is the case with an organ transplant. Nevertheless, the current report (1) offers clear experimental evidence in a large animal model that B-cell targeting in parallel with T-cell inhibition may prevent alloantibody development, and lead to improved long-term graft histology and better small blood vessel patency. Prevention of chronic rejection would represent a major advance for the field of transplantation, and prevention of alloantibody development is more likely to succeed than are strategies to reverse ongoing antibody-mediated graft injury. Acknowledgments 5 The authors are supported by grants # AI_______ to SJK and by ROTRF grant to NI. Address correspondence to: Stuart J. Knechtle, Emory University School of Medicine, 5105 WMB, 101 Woodruff Circle, Atlanta, Georgia 30322, USA. Phone: (404) 7129910; Fax: (404) 727-3660; Email: Stuart.Knechtle@emoryhealthcare.org. References 1. Kelishadi, S., et al. 2010. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J. Clin. Invest. 120:XXX–YYY. 2. Terasaki, P.I. 2003. Humoral theory of transplantation. Am J Transplant 3:665-673. 3. Terasaki, P.I., and Cai, J. 2008. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation 86:377-383. 4. Everly, M.J., et al. 2009. Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant 9:1063-1071. 5. Ramos, E.J., et al. 2007. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant 7:402-407. 6. Zarkhin, V., et al. 2008. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant 8:2607-2617. 7. Kamar, N., et al. 2010. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant 10:89-98. 8. Teeling, J.L., et al. 2006. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 177:362-371. 9. Pescovitz, M.D., et al. 2009. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 361:2143-2152. 10. Hale, D.A., Dhanireddy, K., Bruno, D., and Kirk, A.D. 2005. Induction of transplantation tolerance in non-human primate preclinical models. Philos Trans R Soc Lond B Biol Sci 360:1723-1737. 6 11. Knechtle, S.J., Hamawy, M.M., Hu, H., Fechner, J.H., Jr., and Cho, C.S. 2001. Tolerance and near-tolerance strategies in monkeys and their application to human renal transplantation. Immunol Rev 183:205-213. 12. Sacks, S., Lee, Q., Wong, W., and Zhou, W. 2009. The role of complement in regulating the alloresponse. Curr Opin Organ Transplant 14:10-15. 13. Walters, S., et al. 2009. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol 182:793-801. 14. Bloom, D., et al. 2009. BAFF is increased in renal transplant patients following treatment with alemtuzumab. Am J Transplant 9:1835-1845. 15. Larsen, C.P. et al. 2005. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant 5: 445453. 16. Li, Y., Ma, L., Yin, D., Shen, J., and Chong, A.S. 2008. Long-term control of alloreactive B cell responses by the suppression of T cell help. J Immunol 180:6077-6084. 17. Quezada, S.A., Jarvinen, L.Z., Lind, E.F., and Noelle, R.J. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol 22:307-328. 18. Ferguson, S.E., Han, S., Kelsoe, G., and Thompson, C.B. 1996. CD28 is required for germinal center formation. J Immunol 156:4576-4581. Figure 1. B cell– and antibody-related biologics in transplantation. (I) Anti-CD20 Ab (rituximab), binds and selectively depletes CD20+ B cells. Third generation anti-CD20 mAbs are under development (e.g., ocrelizumab, ofatumumab). (II) BAFF/BlyS inhibitors such as belimumab neutralize B cell activation factor (BAFF) also known as B lymphocyte survival factor (BlyS), or both BAFF/BlyS and APRIL (atacicept, TACI-Ig). (III) Proteosome inhibitors (e.g., bortezomib) reversibly bind to the proteosome and disrupt various cell signaling pathways including NF-B. (IV) Complement inhibitors (AUTHOR: Are these the 3 blue lines with blue circles on the end? Please clarify the figure labeling), such as eculizumab (anti-C5), bind the complement protein C5 (AUTHOR: Please label C5. Only C5b is labeled), leading cessation of complementmediated cell destruction. (AUTHOR: Please include some comment about C3 7 convertase and it’s effect on MAC. Please define MAC. Please also comment on the presence of the C1 complex) (V) CTLA4-Ig/LEA29Y (AUTHOR: Please define LEA29Y in full) (abatacept/belatacept) bind the B7 costimulation molecule. (AUTHOR: Please clarify what the result of this blockade is) (VI) Anti-CD40L (AUTOHR: in the figure the label just reads “Anti-CD40”. Please revise for consistency), binds the CD40L (CD154) costimulation molecule (AUTHOR: please clarify what the effect of this binding is). (AUTHOR: The figure legend is rather stilted. It would be more helpful if it was more narrative about the effects of these molecules in transplantation and graft rejection. Can you also include a brief mention of what part of the figure relates to the results reported in the current report by Kelishadi et al.?) (AUTHOR: once the figure is suitably revised, our in-house illustrator will redraw it prior to publication and you will have the opportunity to review the image at the proof stage and request any more modifications prior to printing) 8