DEPARTMENT OF CHEMISTRY TEACHING LAB EXPERIMENT
advertisement

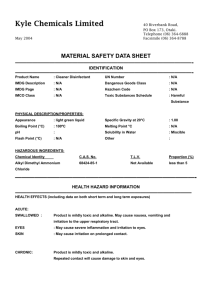
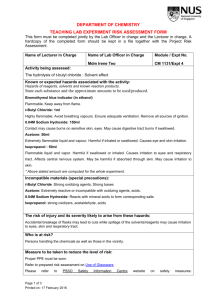
DEPARTMENT OF CHEMISTRY TEACHING LAB EXPERIMENT RISK ASSESSMENT FORM Synthesis lab This form must be completed jointly by the Lab Officer in charge and the Lecturer in charge. A hardcopy of the completed form should be kept in a file together with the Project Risk Assessment. Name of Principal Investigator Name of Lab Officer in Charge Module / Expt No. Dr. Ship Chee Peng Mr. Yeo Boon Hee CM2111 Expt 1 Activity being assessed: 1) Ethanol, 15ml used Flammable liquid and vapour. Causes respiratory tract irritation. Causes severe eye irritation and moderate skin irritation. Ingestion can cause nausea and vomitting. Chronic use can cause serious liver damage. 2) Vanadium pentoxide, 2.0 g used Causes eye irritation. May cause skin irritation. May be fatal if swallowed. High concentrations may cause drowsiness, convulsions, unconsciousness and central nervous system damage. May cause respiratory tract irritation and lung damage. 3) Acetylacetone, 6mL used Flammable liquid and vapour. Very toxic. Causes immediate nausea, and severe irritation of the upper respiratory tract. Can cause vomiting and headache. May be absorbed by skin in toxic amounts with symptoms as in Inhaled. Liquid, vapour and mist are irritating to eyes. Ingestion causes irritation of the mouth, throat, and stomach, with nausea, vomiting. 4) Hydated Sodium carbonate, 20g used Irritation of the nose, and throat may occur due to the irritant nature of sodium carbonate. Causes severe eye irritation and mild skin irritation. 5) Pyridine, 2mL used Flammable liquid and vapour. Vapors cause eye irritation. Splashes cause severe irritation, possible corneal burns and eye damage. Irritates respiratory tract. Affect central nervous system, livers and kidneys. Causes severe irritation, possibly burns, to the skin. 6) Conc. Sulphuric acid, 6ml used Danger! Extremely corrosive! Causes severe burns and eye damage. Strong inorganic acid mists containing sulfuric acid are carcinogenic. Harmful if inhaled. Harmful or fatal if swallowed. Reacts violently with water. Not flammable, but reacts with most metals to form Page 1 of 5 Printed on: 15 February 2016 explosive/flammable hydrogen gas. 7) Ether, 5mL used Extremely flammable liquid and vapour. Slowly can forms explosive peroxides in the presence of light and air and in the absence of inhibitors. May cause headache, nausea, dizziness, drowsiness, and confusion. May be an aspiration hazard. Swallowing or vomiting of the liquid may result in aspiration into the lungs. Known or expected hazards associated with the activity: Hazards of reagents, solvents and known reaction products. State each substance and the approximate amounts to be used/produced. Incompatible materials (special precautions): 1) Conc. sulphuric acid stores away from many materials including water, metals, metal carbides, chlorates, picrates, nitrates, hydroxides, amines, carbonates and other alkaline materials. 2) Pyridine stores away from perchromates, strong acids and strong oxidizers. 3) Chloroform stores away from strong caustics and chemically active metals such as aluminum, magnesium powder, sodium, or potassium; acetone, fluorine, methanol, sodium methoxide, dinitrogen tetroxide, tert-butoxide and triisopropylphosphine. 4) Ethanol stores away from acids, acid anhydrides, or acid chlorides, bromine pentafluoride, disulfuryl difluoride or bromides, strong oxidizing agents, hydrogen peroxide, aqueous ammonia, alkali metals and phosphorus (iii) oxide. 5) Vanadium pentoxide stores away from strong acids. 6) Hydrated Sodium carbonate stores away from acids, magnesium, phosphorus pentoxide, magnesium, phosphorus pentoxide, ammonia and silver nitrate, aluminum, fluorine, lithium and 2,4,6-trinitrotoluene. 7) Acetylacetone stores away from strong oxidizing agents, halogens, strong reducing agents and strong bases. 10) Ether Stores away from strong oxidisers, halogens, interhalogens, sulfur or sulfur compounds, methyl Page 2 of 5 Printed on: 15 February 2016 lithium, peroxyacetic acid and non-metal azides. The risk of injury and its severity likely to arise from these hazards: Spillage and accidental breakage of flasks containing the reagents. Who is at risk? Persons directly in contact with the above stated chemicals. Measure to be taken to reduce the level of risk: Proper laboratory attire and safety measures must always be used in order to reduce the level of risk. . Training prerequisites: Advise students on the hazards of reagents used. Refer to prepared risk assessments on use of glassware, use of fume hoods and use of standard electrical equipment. Use of Glassware Use of Standard Electrical Equipment Level of risk remaining: Low Emergency action if : Spill: 1) Conc. sulphuric acid Neutralise on a dry basis with suitable alkali such as lime follow by flushing with water. 2) Vanadium pentoxide Use a vacuum to collect it and place into container for proper disposal. 3) Pyridine Collect liquid in an appropriate container or absorb with an inert material (e. g. dry sand, earth), and place in a chemical waste container. 4) Ether Eliminate ignition sources. Absorb with solvent absorbent. Fire: 1) Conc. sulphuric acid Page 3 of 5 Printed on: 15 February 2016 Use dry powder fire extinguisher. 2) Vanadium pentoxide Use dry powder fire extinguisher. 3) Pyridine Use dry powder fire extinguisher. 4) Acetylacetone Use dry powder fire extinguisher. 5) Ethanol Use dry powder fire extinguisher. 6) Ether Use dry powder fire extinguisher. Is the experiment suitable for out-of-hours operation? Yes No References if any: Signature of Lab Officer in Charge::……………………………………………………………….. Date:………………………… Signature of Lecturer in Charge:………… …………………………………….. Date:… …………………….. Prepared Risks Assessments for standard equipment and operation are with the kind permission of Dr. Ken MacNeil, School of Chemistry, University of Bristol. Page 4 of 5 Printed on: 15 February 2016 Activity being assessed: Note any activity to be used which entail risk (e.g. use of glass vacuum apparatus, high pressures, high voltage, radiation, high temperatures). Give reference to any special protocols to be followed, and if appropriate attach copies to the risk assessment form. State any additional precautions taken to minimise risk. Known or expected hazards associated with the activity: FOR EACH CHEMICAL, read the MSDS and note:a) Particular hazards (e.g. highly toxic, carcinogenic, corrosive, flammable, pyrophoric, explosive, volatile, dust hazard). Note any dangerous combinations of properties (e.g. volatile and toxic). b) Requirements for safe handling (e.g. fume cupboard, inert atmosphere, low temperature). c) How to dispose of residuals Dispose to drain, with water dilution Neutralise, then to drain with suitable dilution To flammable liquid waste receptacle To non-flammable liquid waste receptacle Keep for recovery/recycling Keep for special disposal later (e.g. heavy metals) Double bag and dispose to dry waste Special procedure (specify) Incompatible materials (special precautions) Note any dangerously incompatible materials and hazards arising from contact of any reagents and substances used with common materials such as paper, benches, hoses, etc. Measures to be taken to reduce the level of risk Include hazards of previously unknown products. Location of work – laboratory, open bench, fume cupboard Level of risk remaining: Likelihood and consequences of any accident or unforeseen events whilst carrying out the activity. When this has been done, choose the appropriate procedure:a) Close supervision and/or attendance of trained first-aider needed. b) Specific approval of supervisor needed. c) Training is needed prior-to or during the operations specified. d) Training is complete and only general laboratory competence required. e) No risk perceived. Emergency action: a) Any special requirements to deal with accidental spillage or leakage. b) What to do in the event of accidental exposure (skin contact, inhalation, etc.). Page 5 of 5 Printed on: 15 February 2016