Key for drug calculations
advertisement

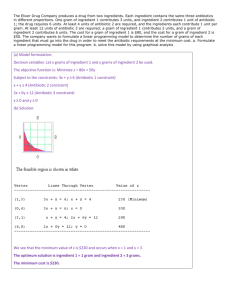
Pharmacology unit 2.2 page 1 Unit 2.2 Homework: Drug calculations Key Corrected! By Elizabeth Kelley Buzbee A.A.S., R.R.T.-N.P.S., R.C.P. Name: Date: 2009 Why do we bother? While drug calculation is not a routine activity for the RCP as it is for the RN, we do, on occasion, have need to calculate drug doses. If a unit dose must be reduced to a smaller size for a patient, or if unit doses have been back ordered and only multi-dose bottles are available, the RCP needs to know how to safely and effectively treat his patient with the proper amount of the drug. The problem with this is that 3 different multi-dose bottles can contain the same drug, each might be in different strengths so that drawing up .5 ml in a syringe from one bottle may be exceeding the amount of drug need while drawing up .5 ml from a different bottle will be much less drug that need. We need to know how to convert drugs from one strength to another in this situation. While one can use the simple ratio formula to calculate drugs, I have found this method to be the quickest way not only to calculate, but to keep the units straight. Drug Strengths Drug Strengths are recorded in several manners: 1. weight/volume, such as gram/ml or micrograms/Liter 2. ratio, such as 1:20,000 or 6:300 3. percent solution, such as .25% solution or 15.5% Because only wt/vol can be used to perform calculations, your first step may be to convert the drug from ratio to wt/vol or from percent to wt/vol. Wt/vol When a liquid drug is mixed in the factory, usually a dry powder or a liquid is dissolved into another liquid such as water or normal saline. The active drug is the solute while the liquid is the solvent-together they are the solution. In the wt/vol method, the solution is listed as solute/solvent. solute / solvent Mg / mL Gram /Liter Micrograms / mL If you have a drug that is recorded as having strength of 5 mg/10 ml, you have 5 mg of solute [active ingredient] that is dissolved into 10 mL of solvent. Complete the following: 1. Example: When you have a drug with a strength of 34 micrograms/1 liter, you have 34 micrograms of solute [active ingredient] that is dissolved into 1 liter of solvent. 2. When you have a drug with a strength of 300 grams/300 ml you have 300 grams of solute [active ingredient] that is dissolved into 300 ml of solvent. 3. When you have a drug with a strength of 2.5 mg/3 ml you have 2.5 mg of solute [active ingredient] that is dissolved into 3 ml of solvent. 4. When you have a drug that is labeled 6 grams/300 ml , you know that you have 6 grams of active ingredient dissolved into 300 ml of solvent. Ratio Pharmacology unit 2.2 page 2 Another common way to express drug strength is to use ratio of solute to solvent. A drug with a ratio of 1:2 has 1 part solute to 2 part solution. Complete the following: 1. Example: When you have a drug with a ratio of 1.25:750, you have 1.25 units of solute [active ingredient] that is dissolved into 750 units of solvent. 2. When you have a drug with a ratio of 25:75, you have 25 units of solute [active ingredient] that is dissolved into 75 units of solvent. 3. When you have a drug with a ratio of 1:10,000, you have 1 unit of solute [active ingredient] that is dissolved into 10,000 units of solvent. 4. When you have a drug with a ratio of 10:100, you have 10 units of solute [active ingredient] that is dissolved into 100 units of solvent. Unfortunately ratios are too inaccurate to use because the ratio contains no units, so over the years, for pharmacology, the ratio was standardized to grams: mL--so if you have a ratio of 1:2, it is understood that you have 1 gram: 2 mL. Complete the following: 1. Example When you have a drug with a ratio of 12.5 : 600, you have 12.5 grams of solute [active ingredient] that is dissolved into 600 mL of solvent. 2. When you have a drug with a ratio of 25:75, you have 25 grams of solute [active ingredient] that is dissolved into 75 mL of solvent. 3. When you have a drug with a ratio of 1:10,000, you have 1gram of solute [active ingredient] that is dissolved into 10,000 mL of solvent. 4. When you have a drug with a ratio of 12:120, you have 12 grams of solute [active ingredient] that is dissolved into 120 mL of solvent. Frequently for most respiratory drugs these units need to be converted from grams: ml to mg: mL 1 gram = 1000 mg Express the following ratios as wt/vol and then convent from grams to mg ratio Grams: mL Mg: mL 1:25 1 gram: 25 mL 1000 mg: 25 mL 1:2.5 1 gram : 2.5 mL 1000 mg: 2.5 mL 1:100 1 gram : 100 ml 1000 mg : 100 mL 2:25 2 gram : 25 ml 2000 mg : 25 ml 6: 300 6 grams: 300 ml 6000 mg: 300 ml 100:100 100 grams: 100 ml 100,000 grams: 100 ml 10:10,000 10 grams : 10,000 ml 10,000 mg: 10,000 ml Pharmacology unit 2.2 page 3 Reduce the ratio Imagine you are a red-shirt on the Enterprise and Spock turns to tell Captain Kirk to tell him that the Romulans out-numbered us 45,000: 450,000. Bones would yell at him. “Simply this, you green-blooded hobgoblin! I’m a doctor—not a calculator!” We have to agree with the good doctor, because--- like Captain Kirk--- we humans understand ratios better if the ratio had been reduced to one in which one of the numbers is 1. When we do this reduction of the ratio, we discover that the Romulans out-number us 1:10. Now, you know you are in serious trouble because you are a red-shirt and know that the red-shirt always dies in this situation. To make the numbers easier to understand and to calculate most ratios need to be reduced to a form in which the solute is 1. To do this we divide both the solute and the solvent by the solute. 600 : 900 = [get rid of extra zeros!] 6 : 9 6 : 9= 6 6 1: 1.5 Reduce the following ratios [watch out for the decimals] ratio Reduce to 1:X 10:25 Grams: mL 1 : 2.5 1 gram : 2.5 ml 120:180 1:1.5 1 gram : 1.5 ml 2:100 1:50 1 gram: 50 mL 2.5:5.0 1: 2 1 gram: 2 mL 600: 300 1: .5 1 gram: .5 mL 500:100 1:.2 1 gram : .2 mL .1:10,000 1: 100,000 1 gram : 100,000 ml Now if we need out solute in mg, we need to reduce adding this step. Reduce the following ratios [watch out for the decimals] ratio Reduce to 1:X Grams: mL .10:2.5 Mg: mL 12.0:18.0 1 :25 1:1.5 1 gram : 25 ml 1 gram: 1.5 ml 1000 mg: 25 ml 1000 mg: 1.5 ml 2:1000 1:500 1 gram : 500 mL 1000 mg:500 ml 20.5:500.0 1:24.3 1 gram : 24.3 mL 1000 mg : 24.3 ml 6.00: 3.00 1:.5 1 gram : .5 ml 1000 mg: .5 ml 15:12,000 1:800 1 gram : 800 ml 1000 mg: 800 ml ratio Reduce to 1:X Grams: mL Mg: mL Pharmacology unit 2.2 page 4 Look at the fourth column of the above table. The problem with converting from grams to mg is that now we have huge numbers again, so it is permissible to again reduce the numbers by dividing both sides by 100 to get the solution easier to understand. Reduce the following ratios [watch out for the decimals] Reduce to ratio Grams: mL Mg: mL Reduce Mg: mL 1:X .10:2.5 1 :25 1 gram : 25 ml 1000 mg: 25 ml 12.0:18.0 1:1.5 1 gram :1.5 ml 1000 mg: 1.5ml 100 mg: 2.5 ml or 10 mg: .25 ml or 1mg : .025 ml 100mg: .15 ml 2:1000 1:500 1 gram:500 mL 1000 mg: 500ml 10mg:5 ml 20.5:500.0 1:24.3 1 gram:24.3 ml 1000 mg: 24.3ml 10mg: .243mL 6.00: 3.00 1: .5 1 gram : .5 ml 1000 mg : .5ml 10mg: .005ml 50.0:1000 1:20 1 gram : 20 ml 1000 mg : 20ml 10mg:0.3=2 ml 15:12,000 1:800 1 gram: 800 ml 1000 mg: 800 ml 10 mg: 8 ml Because the point of the table is to get the number easier to understand it is not always necessary to reduce the solution to this last step, but sometime it is easier if it is done. Convert from ratio to wt/vol. ratio Reduce to Grams: mL Mg: mL Wt /: Volume 1: X 120.5 :500 1: 4.149 1 gram: 4.149 mL 1000 mg: 4.149 100mg/ 4.149 mL mL 16.00: 30.00 1: 1.875 1gram: 1.875 ml 1000mg: 1.875ml 100mg:0.1875 ml 150:100 1:.666 1gram: .666 ml 1000mg:0.666mL 100mg:0.0666 ml 1.5:350 1:233 1gram: 233 ml 1000mg:233 ml 10 mg:.2.33 ml Percentages These are the easiest conversions to wt/vol, because the solute [in grams] is always in a solvent that is 100 ml. so 5% solution is 5grams/100 mL. Because there will be so many zeroes in this answer, reduction might help. convert these percentages to wt/vol percent ratio Wt / vol Reduce 100 mL to 1mL 50% 50:100 50gram/100mL .5 gram/1 ml or or Pharmacology unit 2.2 page 5 4.5% 4.5 : 100 12.5% 12.5: 100 75:100 50,000 mg in 100 mL 500 mg/1 ml 4.5 grams: 100 ml or 4,500 mg:100 ml 12.5 grams: 100 ml 12,500 mg: 100 ml 75gram: 100 ml 45mg: 1ml 125 mg: 1 ml .75grams: 1ml 75% .5:100 .5% .5grams:100ml 500 mg:100 ml 5 mg:1 mL Calculation of drug dosages As stated at the beginning of this lecture, we must convert our drug labeled in Percentage or in ratio to the wt/vol format before we can calculate a drug dose. We are now ready for the drug calculation. These are the steps: 1. discover the question being asked and the units in which the answer must appear 2. conversion to wt/vol 3. set up the equation 4. solve the equation 1. discover the question being asked and the units in which the answer must appear In word questions, sometimes the hardest thing to determine is the actual information the situation asks for. In drug calculations, generally, there are only two possible bits of information need: [1] you have a multi-dose bottle and need to know how many mL [or Liters] you must draw up. [2] you need to know in grams [or mg] how much active ingredient is present in a given volume [mL or Liters] of a solution. After you have determined which question has been asked, you find out what units are required. This will determine how much you reduce your figures prior to calculation or if you even need to reduce it. For example, you are asked how much active ingredient is in a bottle labeled 1: 3. You are not asked how many mg, so you could leave the answer in grams rather than go through the steps of converting from grams to mg. Another example is that the doctor has ordered 2 mg of a drug. You have a bottle labeled 2% so you will have to convert this 2% into 2000 mg/100 ml and reduce it to 20 mg/1 ml in order to discover how many ml will yield 2 mg. 2. conversion to wt/vol Once you have discovered the information you need, you convert the unit to wt/vol, and then reduce as needed to get the units the question requires. For example, you are asked how much active ingredient is in a bottle labeled 5%. You are not asked how many mg, so could convert from 5% to 5gram/100 ml in order to do the next step. But if your doctor has ordered 2.5 mg from a bottle labeled .5%, you must first convert the .5% to .5grams/100 ml and then to 500 mg/100 ml and even 5 mg/1 ml to calculate your answer. If your doctors orders 2.5 mg from a bottle labeled 12:75, you would be better to reduce the ratio from 12:75 into 1:6.25, then 1 gram: 6.25 ml, before converting to 1000 mg/6.25 mL. Pharmacology unit 2.2 page 6 3. set up the equation While you could answer a lot of these questions by just setting up a ratio, some folks use the following formula: A. doctor’s order [include the unit] B. dose on hand [in units you need] Nothing goes here C. Dose on hand [in same units as the doctor’s order.] We multiply A and B, the divide the answer by C. Example A: Your doctor has ordered 2.5 mg from a bottle labeled .5%. We know that .5% is .5gram/100ml but we need mg so we convert to 500 mg/100 ml A. 2.5 mg B. 100 ml Nothing goes here C. 500 mg We multiply A and B, the divide the answer by C. [2.5 mg x 100 ml] 500 mg [the mg cancel out and we are left with ml] 250.0 ml /500 = .5 .5 ml need to be drawn up to give 2.5 mg Example B: you are asked how much active ingredient is in a bottle labeled 25% if you draw up 5 ml. you convert 2.5% to 2.5 grams/100 mL, because the question was not asked in terms of mg, we can stay with grams. A. 5 ml B. 2.5 grams Nothing goes here C. 100 mL. We multiply A and B, the divide the answer by C. 5 ml x 2.5 grams 100 ml [the ml cancel out and we are left with grams] 12.5 grams / 100 = .125 grams of active ingredient are present in 5 ml of 25% solution Now it is your turn. Answer the following questions: 1. How much do you draw up to administer 3.0 mg of a 10% solution? 10%= 10 gram:100 ml =10,000 mg :100 ml = 100mg:1 ml 3 mg x 1 ml 100 mg 3/100= .03ml Pharmacology unit 2.2 page 7 2. How much do you draw up to administer 20 mg of a .5% solution? .5% = .5grams:100ml=500 mg: 100ml = 5mg:1 mL 20 mg x 1 ml 5mg 20/5 = 4 ml 3. How much do you draw up to administer 4.6 grams of a 25% solution? 25% = 25grams :100ml 4.6 grams x 100 ml 25 grams 460/25 = 18.4 ml 4. How much do you draw up to administer 2.5 grams of a 10:10,000 solution? 10:10,000 = 1:1000= 1 gram: 1000 ml = 2.5 grams x 1000 ml 1 gram 2500/1 = 2500 ml or 2.5 Liters 5. Your doctor orders 3 mg from a drug labeled 3:600 solution. How much do you draw up? 3 grams: 600 ml = 1 gram: 200ml = 1000 mg: 200 ml = 10mg : 2ml =5mg: 1 ml 3 mg x 1 ml 5 mg 3/5 = .6 ml 6. How much do you draw up to administer 45.3 mg of a 1:3 solution? 1:3 = 1 gram: 3ml = 1000 mg: 3ml 45.3 mg x 3 ml 1000 mg 135.9 /1000 = .1359 ml How much do you draw up to administer 3 mg of a 3.5 mg: 1ml solution? 3 mg x 1 ml 3.5 mg 3/3.5 = .857 ml How much do you draw up to administer 5 grams of a 60 gram:10 ml solution? 5 grams x 10 ml 60 grams 50/60 = .8333 ml 7. 8. 9. How much do you draw up to administer .25 gram of a 5.5 gram:100 ml solution? .25 grams x 100 ml 5.5 grams 25/5.5 = 4.54 ml Pharmacology unit 2.2 page 8 10. Your doctor orders 3 mg of a drug that is labeled 4grams/ml. How much do you draw up? 4 grams: 1 ml = 4000 mg / 1 ml 3 mg x 1 ml 4000 mg 3/4000 = .00075 mL 11. You want to know how much active ingredient is found in 50 ml of a 1:10,000 solution 1:10,000 = 1000 mg : 10,000 ml = 1 mg/ 10ml 50ml x 1mg 10ml 50/10 = 5 mg 12. You want to know how much active ingredient is found in 1.550 ml of a 50:250 solution 50: 250 = 1:5 = 1gram : 5 ml 1.55ml x 1 mg 5ml 1.55/5 = .31 mg 13. You want to know how much active ingredient is found in 150 ml of a 1:30 solution. 1:30 = 1 gram/30 ml 150 ml x 1 gram 30ml 150/30 = 5 grams 14. You want to know how much active ingredient is found in 100 ml of a 2.5% solution 2.5%= 2.5 grams: 100 ml 100 ml x 2.5 gram 100 ml 250/100 =2.5 g 15. You want to know how much active ingredient is found in 50 ml of a 38% solution 38% = 38 grams : 100ml 50 ml x 38 gram 100 ml 1900/100 = 19 gram 16. You want to know how much active ingredient is found in 10 ml of a 50% solution 50% = 50 grams/100 ml = 5 grams/10 ml 10 ml x 5 gram 10 ml 50/10 5 gram Pharmacology unit 2.2 page 9 17. You want to know how much active ingredient is found in 1.5 ml of a 300mg/ 1ml solution 300 mg/1 ml 1.5 ml x 300mg 1 ml 450/1 = 450 mg 18. You want to know how much active ingredient is found in 250 ml of a 150mg/ 10ml solution 150 mg/10 ml = 15 mg/1ml 250 ml x 15 mg 1 ml 3750 mg or 3.75 grams 19. You want to know how much active ingredient is found in 65 ml of a 3.5 mg/ 1ml solution 65 ml x 3.5 mg 1 ml 227.5 mg