Chap 1: Atomic Scale Structure of Materials
advertisement

Chap 1: Atomic Scale Structure of Materials
This teaching and learning package provides an introduction to crystalline, polycrystalline and
amorphous solids, and how the atomic-level structure has radical consequences for some of
the properties of the material. It introduces the use of polarised light to examine the optical
properties of materials, and shows how a variety of simple models can be used to visualise
important features of the microstructure of materials
Aims
Introduction
Single crystals: Shape and anisotropy
Single crystals: Mechanical properties
Single crystals: Optical properties
Polycrystals
Defects
Summary
Questions
On completion of this tutorial you should:
know the differences between single crystal, polycrystalline and amorphous solids
be able to identify the characteristic features of single crystals and polycrystals
understand the nature of crystal defects
appreciate the use of polarised light to examine optical properties
1.1 Introduction
The fundamental difference between single crystal , polycrystalline and amorphous solids is
the length scale over which the atoms are related to one another by translational symmetry
('periodicity' or 'long-range order'). Single crystals have infinite periodicity, polycrystals have
local periodicity, and amorphous solids (and liquids) have no long-range order.
An ideal single crystal has an atomic structure that repeats periodically across its
whole volume. Even at infinite length scales, each atom is related to every other
equivalent atom in the structure by translational symmetry.
A polycrystalline solid or polycrystal is comprised of many individual grains or
crystallites. Each grain can be thought of as a single crystal, within which the atomic
structure has long-range order. In an isotropic polycrystalline solid, there is no
relationship between neighbouring grains. Therefore, on a large enough length scale,
there is no periodicity across a polycrystalline sample.
Amorphous materials, like window glass, have no long-range order at all, so they have
no translational symmetry. The structure of an amorphous solid (and indeed a liquid)
is not truly random - the distances between atoms in the structure are well defined and
similar to those in the crystal. This is why liquids and crystals have similar densities both have short-range order that fixes the distances between atoms, but only crystals
have long-range order.
The range of crystalline order distinguishes single crystals, polycrystals and amorphous solids. The
figure shows how the periodicity of the atomic structure of each type of material compares.
Many characteristic properties of materials, such as mechanical, optical, magnetic and
electronic behaviour, can be attributed to the difference in structure between these three
classes of solid
1.2 Single crystals: Shape and anisotropy
A single crystal often has distinctive plane faces and some symmetry. The actual shape of the
crystal will be determined by the availability of crystallising material, and by interference
with other crystals, but the angles between the faces will be characteristic of the material and
will define an ideal shape. Single crystals showing these characteristic shapes can be grown
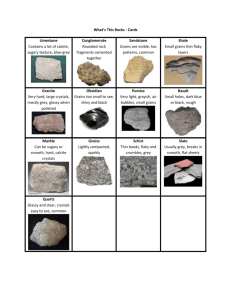
from salt solutions such as alum and copper sulphate.
Gemstones are often single crystals. They tend to be cut artificially to obtain aesthetically
pleasing refractive and reflective properties. This generally requires cutting along
crystallographic planes. This is known as cleaving the crystal. A familiar example is diamond,
from which decorative stones can be cleaved in different ways to produce a wide range of
effects.
To see a variety of symmetrical naturally formed minerals, visit the mineral galleries website.
Consider the following three-dimensional shapes:
Cube: 6 identical squares
Tetrahedron: 4 identical equilateral triangles
Octahedron: 8 identical equilateral triangles
Rhombohedron: 6 identical parallelograms with
sides of equal length
These are examples of regular polyhedra , composed of identical two-dimensional shapes,
with edges are all the same length. You can make your own cube, octahedron and tetrahedron
by printing the following pages and following the instructions on them.
Patterns for cube and tetrahedron
Pattern for octahedron
These three shapes are the most important in materials science, and you should be very
familiar with them!
The symmetry exhibited by real single crystals is determined by the crystal structure of the
material. Many have shapes composed of less regular polyhedra, such as prisms and
pyramids.
Hexagonal prism: 2 hexagons and 6 rectangles
Square-based pyramid: 4 triangles and a square
Not all single crystal specimens exhibit distinctive polyhedral shapes. Metals, for example,
often have crystals of no particular shape at all.
These quartz specimens show a range of shapes typically exhibited by crystals. (Click on an image to
see a larger version.)
Most single crystals show anisotropy in certain properties, such as optical and mechanical
properties. An amorphous substance, such as window glass, tends to be isotropic . This
difference may make it possible to distinguish between a glass and a crystal. The
characteristic shape of some single crystals is a clue that the properties of the material might
be directionally dependent. The properties of polycrystalline samples can be completely
isotropic or strongly anisotropic depending on the nature of the material and the way in which
it was formed.
1.3 Single crystals: Mechanical properties
Gypsum can be cleaved along particular crystallographic planes using a razor blade. The
bonding perpendicular to these cleavage planes is weaker than that in other directions, and
hence the crystal breaks preferentially along these planes. Quartz and diamond do not have
such distinct cleavage planes, and so cleaving these crystals requires much more effort and
care.
There are distinct planes in the gypsum structure, with no bonding between them. These are
the cleavage planes. It is much more difficult to cleave gypsum along planes other than these.
In contrast, all of the planes in the quartz structure are interconnected and the material is much
more difficult to cleave in any direction. This is a demonstration of a way in which the crystal
structure of a material can influence its mechanical properties.
Certain crystals, such as gypsum, can be cleaved with a razor blade along particular
crystallographically-determined planes. (Click on image to view larger version.)
Glass is impossible to cleave. As an amorphous substance, glass has no crystallographic
planes and therefore can have no easy-cleavage directions. Glassy materials are often found to
be mechanically harder than their crystalline equivalents. This is an example of how
mechanical properties of crystals and amorphous substances differ.
1.4 Single crystals: Optical properties
Quartz crystals are birefringent , so they exhibit optical anisotropy. Consider plane polarised
light passing through a birefringent crystal. Inside the crystal, the light is split into two rays
travelling along permitted vibration directions (p.v.d.s). The two rays are subject to different
refractive indices, so the light travelling along each p.v.d. reaches the opposite side of the
crystal at a different time. When the two rays recombine, there is a phase difference between
the two rays that causes the polarisation state of the transmitted light to be different from that
of the incident light.
Optical anisotropy in thin samples can be observed by placing the sample between crossed
polarising filters in a light box. The bottom filter, between the light source and the sample, is
called the polariser . The top filter, between the sample and the observer, is called the analyser
. The polariser and analyser have polarising directions perpendicular to one another.
The apparatus used for examining optical anisotropy consists of a white-light source, two polarising
filters and a frame to hold them apart so creating a working space.
When no sample is in place the light that reaches the analyser is polarised at 90° to the
analyser's polarisation direction, so no light is transmitted to the observer. When a quartz
sample (with favourable orientation, see later) is placed between the filters, the crystal
changes the polarisation state of the light that is transmitted through it. When this light
reaches the analyser, some component of it lies parallel to the polarisation direction of the
analyser, and therefore some light is transmitted to the observer.
If a quartz slice shows optical anisotropy, the intensity of light transmitted through the
analyser varies as a function of the angle of rotation of the quartz sample in the plane of the
filters. At certain orientations, no light is transmitted. These 'extinction positions' are found at
90° intervals.
When the same experiment is done using a piece of glass, it is found that light is not
transmitted for any orientation. This is because the glass is optically isotropic, and does not
change the polarisation state of the light passing though it.
In quartz, there is one direction of propagation for which no birefringence is observed. If a
sample is cut so that the incident light is parallel to this direction, the sample behaves as if it is
optically isotropic and no light is transmitted. The crystallographic direction that exhibits this
property is known as the optic axis.
When the quartz sample is cut so that the incident light is parallel to the optic axis, no light is
transmitted in any orientation.
This experiment demonstrates that some single crystals, such as quartz, show anisotropic
optical properties. The phenomenon depends on the crystallographic orientation of the crystal
with respect to the incident light. Amorphous materials like glass have no 'distinct' crystal
directions, so anisotropic properties are generally not observed.
1.5 Polycrystals
Single crystals form only in special conditions. The normal solid form of an element or
compound is polycrystalline. As the name suggests, a polycrystalline solid or polycrystal is
made up of many crystals. The properties of a polycrystal are notably different from those of a
single crystal. The individual component crystallites are often referred to as grains and the
junctions between these grains are known as grain boundaries .
The size of a grain varies according to the conditions under which it formed. Galvanised steel
has a zinc coating with visibly large grains. Other materials have much finer grains, and
require the use of optical microscopy.
In galvanised steel, the grains are big enough to be seen unaided. The plate measures 5 cm across.
In many other metals, such as this hypoeutectoid iron-carbon alloy, the grains may only be seen under
a microscope. (Click on image to see larger version.)
These photographs show a polycrystalline sample of quartz mixed with feldspar in which the
grains all have optically anisotropic properties. Between the crossed polarisers, each grain
allows transmission of light at a slightly different point in the rotation. This gives the strange
effect seen here.
This polycrystal contains randomly oriented grains that allow transmission at different angles.
Consequently different regions of the polycrystal are seen in these two photographs. (Click on the
images to view larger versions.)
The three-dimensional shape of grains in a polycrystal is similar to the shape of individual
soap bubbles made by blowing air into a soap solution contained in a transparent box.
The surface between bubbles is a high-energy feature. If the area of the surface is decreased,
the overall energy of the system decreases, so reduction of surface area is a spontaneous
process. If all the bubbles were the same size, the resulting structure would be a regular closepacked array, with 120° angles between the surfaces of neighbouring bubbles.
In practise, bubble growth can occur because the surface area of a few large bubbles is lower
than that of many small bubbles. Large bubbles tend to grow, and small bubbles tend to
shrink. The bubbles are therefore different sizes so there are large deviations from the closepacked structure. On average, however, three bubbles meet at a junction, and the angle
between the bubble surfaces is usually within a few tens of degrees of 120°.
The curvature of the surfaces is also important. Surfaces with a smaller radius of curvature
have a higher energy than those with a larger radius of curvature. As a result, some small
bubbles cannot shrink and disappear, even though the surface area would decrease if they did
so. This is because the curvature of the boundaries, and the associated energy, would be too
high.
In a real polycrystal, the grain boundaries are high-energy features, similar to the surfaces
between bubbles. The soap froth is a very good model for the grain structure of a simple
polycrystalline material, and many similar features can be observed in the two systems. The
soap bubbles are analogous to the grains, and the surfaces of the bubbles are analogous to the
grain boundaries. Compare the photographs of the soap bubbles with the micrograph of a
polycrystalline material that has been etched to reveal the grain boundaries.
The packing of soap bubbles is somewhat similar to the packing of crystals - both systems seek to
minimise their surface area. Note the angles at the junctions of grain boundaries. (Click on images to
view larger versions.)
The grains of this hypereutectoid iron-carbon alloy are packed in a similar way to the bubbles in the
previous photographs. (Click on image to view larger version.)
Grain boundaries in a polycrystalline solid can relax (move in such a way to decrease the total
energy of the system) when atomic rearrangement by diffusion is possible. In real materials,
many other effects can influence the observed grain structure.
1.6 Defects
Within a single crystal or grain, the crystal structure is not perfect. The structure contains
defects such as vacancies , where an atom is missing altogether, and dislocations , where the
perfection of the structure is disrupted along a line. Grain boundaries in polycrystals can be
considered as two-dimensional defects in the perfect crystal lattice. Crystal defects are
important in determining many material properties, such as the rate of atomic diffusion and
mechanical strength.
We can use a "shot model" to get a picture of crystal defects. The model consists of many
small ball bearings trapped in a single layer between two transparent plates. They tend to
behave like the atoms in a crystal, and can show the same kind of defects.
When the shot model is held horizontally, so that the balls flow freely, the resulting structure
is similar to a liquid.
Shot model held horizontally. The balls form a liquid-like structure. (Click on image to view larger
version.)
As the model is tilted towards the vertical, the balls pack closely together. This represents
crystallisation. One or two balls may be suspended above the main body by electrostatic
forces: this is comparable to the vapour found above the crystal.
In some places the balls form close-packed regions. Tapping of the model causes minor
rearrangements of the balls, especially at the top of the "solid" region. This is similar to
diffusion, in which case the tapping is analogous to thermal activation. Occasionally, the
"diffusion" process may cause two grains to join together, or for some grains to "grow". The
following image sequence shows the behaviour of the shot model as it is rearranged by
tapping, starting from a polycrystalline state with many small grains and ending with much
larger grains. Note the presence of vacancies in the structure.
With great care, it may be possible to create a single crystal, as all the balls form a single
pattern. Note that diffusion occurs mainly near the top of the balls: those towards the middle
and bottom do not easily move, as the photographs show.
Even in a single crystal, or large-grained sample, there are still vacancies, as the shot model
shows. The reason for this involves entropy: at all finite temperatures, there will be some
disorder in the crystal.
The balls within a grain arrange themselves into close-packed planes. In metals, close-packing
of atoms is a very common structure. This pattern is typical of hexagonal close-packed and
cubic close-packed lattices. Note that in this 2-D model, each ball touches six others. In a 3-D
crystal, such as a real one, each ball would also touch three on the plane above, and three on
the plane below.
A close-packed plane.
In the shot model, the balls are normally arranged in to a polycrystalline form, shown
schematically below:
A polycrystal will typically have crystalline regions (grains) bounded by disordered grain boundaries.
These boundaries are marked in the picture on the right.
Note that the packing of atoms at the grain boundaries is disordered compared to the grains.
At a grain boundary, the normal crystal structure is obviously disturbed, so the boundaries are
regions of high energy. The ideal low energy state would be a single crystal.
Polycrystals form from a melt because crystallisation starts from a number of centres or nuclei
. These developing crystals grow until they meet. Since they are not usually aligned before
meeting, the grains need not necessarily be able to fit together as a single crystal, hence the
polycrystalline structure.
After crystallisation the solid tends to reduce the boundary area, and hence the internal
energy, by grain growth. This can only happen by a process of atomic diffusion within the
solid. Such diffusion is more rapid at a higher temperature since it is thermally activated .
1.7 Summary
The focus of this package is the difference between single crystals, polycrystals and
amorphous solids. This is explained in terms of the atomic scale periodicity: single crystals
are periodic across their entire volume; polycrystals are periodic across individual grains;
amorphous solids have little to no periodicity at all.
The different atomic structures can have effects on the macroscopic properties. A single
crystal may exhibit anisotropy - we have seen mechanical anisotropy of gypsum, and optical
anisotrpy of quartz. Polycrystals may also be anisotropic within each grain, as seen when the
polycrystalline quartz-feldspar mix was placed between the crossed polarisers. Amorphous
solids do not have anisotropic mechanical or optical properties, since they are isotropic on the
atomic scale.
Defects may exist in all structures, even single crystals. They include vacancies and grain
boundaries, where the regular repeating structure is disrupted.