Reverse Reactions: Photosynthesis and Cellular Respiration
advertisement

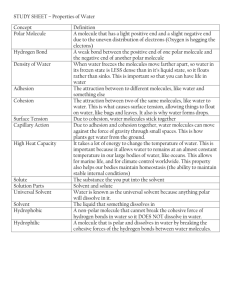
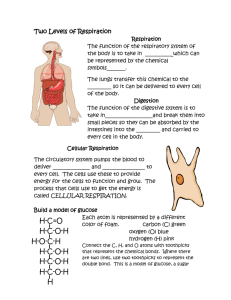
Reverse Reactions: Photosynthesis and Cellular Respiration Purpose Use Lego bricks to build molecules of carbon dioxide, water, oxygen and glucose in order to model the chemical reactions of photosynthesis and cellular respiration, to understand how energy is stored and released in plants, and how plants use these compounds to produce biomass and carry on all life functions. Key Questions What do plants do with the “food” they produce via photosynthesis? Where does a plant get its “energy” and what does it do with this energy? Where does a tree get its mass? Student Outcomes 1. Students will be able to write or diagram the chemical reactions for photosynthesis and cellular respiration. (See equations below.) 2. Students will demonstrate understanding of how plants release the energy stored in the chemical bonds of the glucose the plant produced as a result of photosynthesis. 3. Students will demonstrate understanding of what plants do with the food they manufacture, including how biomass is produced. 4. Students will demonstrate understanding of how plants provide energy for consumers in its role as a producer. State Standards 1. Organisms in ecosystems exchange energy and nutrients among themselves and with the environment. As a basis for understanding this concept: a. Students know energy entering ecosystems as sunlight is transferred by producers into chemical energy through photosynthesis and then from organism to organism through food webs. Key Concepts 1. The capture and use of energy in living systems is dominated by two processes: photosynthesis and respiration. 2. Plants take in carbon dioxide and water, and using the energy from the sun, these two gases combine in a chemical reaction to produce glucose and oxygen. The sun’s energy is stored as chemical energy in the bonds of the glucose. This process of producing glucose is called photosynthesis. It is represented by the equation: 6 CO2 + 6 H2O + energy (from sunlight) C6H12O6 + 6 O2 3. Plants need energy to carry on all of life’s processes: growth, reproduction, gas exchange, make food, respond to stimuli, movement, and excrete waste. Plants use oxygen in the air (or water) to turn their food (glucose) into energy. This process of using oxygen to release energy from food is called cellular respiration. 4. Cellular respiration occurs in the mitochondria of the plant cell. The glucose molecule, using oxygen, is broken apart and turned back into carbon dioxide and water, the same types of molecules that originally combined to make the glucose. The solar energy that was used to make the glucose molecule is released to the cell as chemical energy. This process of breaking down glucose for energy is called cellular respiration. It is represented by the equation: C6H12O6 + 6 O2 6 CO2 + 6 H2O + energy 5. Sugars created in photosynthesis can be later converted by the plant to starch for storage, or it can be combined with other sugar molecules to form specialized carbohydrates such as cellulose, or it can be combined with other nutrients such as nitrogen, phosphorus, and sulfur to build complex molecules such as proteins and nucleic acids. Cellulose is probably the single most abundant organic molecule in the biosphere. Materials Procedure 1 Task Card 18 Lego 2x2 bricks in one color (white) 12 Lego 2x2 bricks in a second color (red) 6 Lego 2x2 bricks in black 1. Have the Lego bricks separated into individual bricks grouped by color. 2. Write the chemical formula for water on the board. (H2O) Ask students what they think H and O stand for. (H is for hydrogen and O is for oxygen). A water molecule is made out of oxygen atoms and hydrogen atoms. Have students look at the chemical formula for water. Ask them how many hydrogen atoms and how many oxygen atoms combine (bond) together to make a molecule of water (2 hydrogen, 1 oxygen) 3. Tell students that each Lego represents an atom, an individual building block of matter. Each color represents atoms of a different element. Post the following color/element combinations on the board: White = oxygen Red = hydrogen Black = carbon 4. Ask the students to assemble the blocks to represent a water molecule. Explain that in order to be considered a molecule, the atoms “blocks” must be “bonded,” or snapped together. 5. Write the chemical formula for carbon dioxide (CO2) on the board. Ask students to assemble the blocks to represent a molecule of carbon dioxide. 6. Ask students to make 6 water molecules and 6 carbon dioxide molecules. The students now have the molecules a plant uses (H2O and CO2) to make glucose. 7. Write the chemical formula for glucose (C6H12O6). Using only the 6 water molecules and 5 carbon dioxide molecules the students have made, challenge them to make a glucose molecule. How many glucose molecules can they make with the blocks they have? (Answer: 1) 8. What types of atoms are left after the glucose molecule has been made? (Answer: 12 oxygen atoms). Oxygen in the air does not like to float around by itself. Oxygen forms a diatomic molecule (two atoms of the same element bonded together). Ask the students to make as many diatomic oxygen molecules as they can. They should be able to make six O2 molecules. 9. Students now have glucose and oxygen, the products of photosynthesis. Ask the students to think about what the plant does with these products. Where does the glucose molecule go and where does the oxygen molecules go? (The plant needs glucose and will store it in its roots and stems in the form of starch. Later, the plant will burn the glucose molecule for the energy the plant requires to carry on all of the life processes, including growth. The oxygen molecule is a waste product and is given off as a gas through the stomata in the leaves.) 10. Ask the students to reflect in their science notebooks about what they have done and learned to this point. They should be able to write the photosynthesis reaction. They may draw a picture of the reaction or what is taking place in the plant. They may ask questions about what they still want to know or write something new that they have learned. 11. Ask students to look at the glucose molecule. Explain to them that the glucose molecule holds a lot of chemical energy in the bonds that were created to hold the individual atoms together to form the molecule. 12. Ask the students what will happen when the plant or animal needs to use the glucose molecule for energy? (The plant or animal will send the glucose to the mitochrondia in the cell. The oxidation of the glucose by organisms is called respiration. This process occurs in both plants and animals. Respiration in plants releases the energy required for all life processes. It requires 6 O2 molecules to break the bonds of the glucose molecule, thereby releasing the energy it held in its chemical bonds. 6 CO2 and 6 H2O molecules are waste products. YES, plants DO GIVE OFF CO2 when they respire. Respiration, which produces carbon dioxide and water, is constantly being balanced against photosynthesis in nature and in agriculture. If the carbon dioxide fixed via photosynthesis were exactly the equal to the carbon dioxide produced by respiration in the plant, it would contribute nothing to the buildup of biomass in the plant. Photosynthesis must exceed respiration by a considerable amount to accumulate biomass. In agriculture, farmers attempt to achieve a photosynthesis/respiration ratio as high as 40:1. www.biologie.uni-hamburg.de) 13. Ask students to think about what we need for energy. (Food and oxygen). How do we take in the items we need (ingest via the mouth; breathe via the lungs). Where does the reaction that gives us our energy take place? Why do we breathe out CO2? 14. Students can simulate the increase in biomass by snapping several glucose molecules together, forming specialized carbohydrates called cellulose, which gives plants their structure. Energy from cellular respiration is needed for the plant to combine glucose molecules into cellulose, which is a form of growth. 15. By combining other nutrients such as nitrogen, phosphorus with glucose, the plant can build complex molecules such as proteins and nucleic acids. This can be easily modeled by adding a 4th and 5th color Lego brick to the glucose molecule.