MOLECULES, CELLS AND DISEASE – Introduction to cells
advertisement

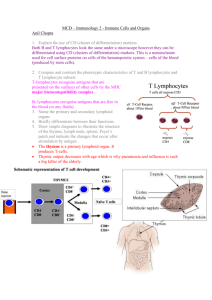
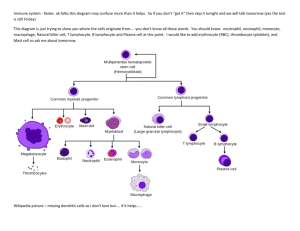
Lecture 1: Introduction to immunology 1) Explain the importance of immunology for human health 2) Outline the basic principles of immune responses, and their time scales in which they occur The immune system recognises non-self that enters the body and responds to it Innate immune system works rapidly (minutes) and has a broad specificity Adaptive immune system takes longer (days) and has exquisite specificity 3) Define the terms antigen, antibody, B lymphocyte, T lymphocyte, active and passive immunity, primary and secondary immune responses Antigen = molecules which react with antibodies. However not all antigens induce antibody production, only those which are termed immunogens Antibody = the product of humoral; immune response. They bind specifically to molecules called antigens. They are found in bloodstream and body fluid. Lymphocytes = mononuclear cells, which are part of the leukocyte cell lineage. Subdivided: T cells (thymus derived); B cells (bone marrow derived). Lymphocytes express receptors on their surface to enable recognition of a specific antigen. T cells = originate in the thymus. They recognise antigen antigen presented at the cell surface by surface by MHC molecules. Surface makers found on T cells are CD3, CD4 and CD8. ( The T cell antigen receptor is NOT a membrane bound antibody – but a distinct molecule = T cell antigen receptor. B cells originate in the bone marrow. They recognise free antigen in the body fluids. Surface markers associated with B cells are CD19 and surface immunoglobin ClassII MHC. (the B cell antigen receptor is a membrane bound antibody = surface immunoglobulin) Active immunity = is the induction of an immune respose by the introduction of an antigen Passive immunity = is immunity gained without antigen induction i.e. by the transfer of antibody or immune serum into a naïve recipient. (this is a temporary protection, immunoglobulins are not everlasting, but however they have an immediate effect) Primary response = the response made by naïve lymphocytes when they first encounter their specific antigen. Secondary response = the response made by memory lymphocytes when they re-encounter their specific antigen. Lymphocytes make up 20-40% of the circulating WBCs, they express membrane molecules called CD (cluster of differentiation) molecules. They are essentially inactive until they encounter an antigen. 4) Outline the concept of clonal selection, and its role in immune responses. T and B cells produced in the primary lymphoid organs are released into the peripheral lymphoid pool. Those which meet their specific antigen are selected for expansion and the production of effector and memory cells. Those which do not meet their antigen will eventually die. The size of the peripheral lymphoid pool is regulated by homeostatic mechanisms. Primary response: Produce antibody Primary Response B cells Antigen Function T cells Naïve Lymphocytes Selection Activation Clonal Expansion Cytotoxic T cell Kills infected cells Helper T cell Helps B cell make antibody Or helps T cell become cytotoxic When the antigen is cleaned up and removed, the response stops, but some of the cells from this clonally expanded population survive as memory cells, which are used in the secondary response. Secondary response: The specific immune response to a pathogen is more effective on re-exposure to the pathogen compared with the initial response. This ‘more effectiveness’ is characterised by a response which is far greater in magnitude and more rapid than the primary response (faster, more intense, more IgG than IgM in secondary response): Page 1 of 11 Primary Lag 4-7 days peak 7-10 days Ig M Secondary Lag 1-3 days peak 3-5 days Ig G Higher affinity Requires T-help 100-1000 higher Lasts longer Antibody responses Serum Ig level to antigen 2o o 1 Ig M Ig G but some Ig M Time Antigen Antigen 5) Appreciate that the physical organisation of the immune system is important for its function. The site of maturation and initiation of adaptive immune response are: Primary lymphoid organs = thymus; bone marrow Secondary lymphoid organs = lymph nodes; spleen; mucosal associated lymphoid tissue (malt) Since there are million of lymphocytes in the body and thus millions of different antigen receptors, the way in which an antigen meets its receptor is by: Constant movement around the body Tracking through specialist structures whose purpose is to bring together pathogen and responding lymphocyte (these are the secondary lymphoid tissues) Lecture 2: Immune cells and organs 1) Name the primary and secondary lymphoid organs and briefly differentiate between their functions; draw simple diagrams to illustrate the structure of the thymus, lymph node, spleen, Peyer’s patch and indicate the changes that occur after stimulation by antigen. Primary lymphoid organs are the major sites of lymphopoiesis. Here lymphoid stem cells differentiate into mature functional lymphocytes.the two primary lymphoid tissues are: the thymus and the bone marrow The thymus is bi-lobed in mammals, located in the thorax. Each lobe is organised into lobules and in each lobules are histologically defined regions of cortex and medulla. The cortex contains immature thymocytes some of which are selected to become mature thymocytes in the medulla. There is a great deal of cell death in the thymus and only a small percentage of the cells exit the thymus into the peripheral T cell pool. The mammalian thymus atrophies with age and areas of active T cell production are replaced with adipose tissue. T cell progenitors enter the thymus glnad as immature thymocytes and emerge as mature, antigen specific, immunocompetent T cells. Schematic representation of T cell development THYMUS Bone marrow CD4+ CD8- Cortex Medulla - CD4 CD8- CD4+ CD8+ Naïve T cells CD4CD8+ The bone marrow In the foetus the liver is active prior to most bones becoming active sites of production. In later life active sites may include spongy regions at the end of long bones; also vertebral bones, sternum, ribs, flat bones o the cranium and pelvis. Bone marrow produces stem cells and B lymphocytes. Those stem cells destined to become T lymphocytes migrate to the thymus throughout life. For B cells, differentiation is centripetal with the stem cells under the bone and the most mature phases of the B cell pathway found nearer the centre of the marrow. Shaded areas = active sites Secondary lymphoid organs provide an environment in which lymphocytes can interact with antigen and with other lymphocytes. They have special vascular adaptations to recruit lymphocytes from the blood. The spleen contains two main types of tissue, the red pulp and the white pulp. The red pulp acts as a general filter and the white pulp is the lymphoid tissue and constitutes the major initiator of responses to blood borne antigens. Around the central arteriole are concentric cuffs of lymphoid tissues = the periarterial lymphatic sheath. The region nearest the arteriole is a T cell zone. Periodically, there are B cell follicles, either primary or secondary and around this is the marginal zone which seems to be the primary site of entry of B and T cells into the white pulp. Page 2 of 11 Marginal Zone Capsule B cell area Trabecular vein Trabecular artery Central arteriole T cell area Human lymph nodes are 1-15 mm across, are round or kidney shaped and have an indentation at the hillus where the blood vessels (BV) enter and leave the node. Lymph arrives at the lymph node through several afferent vessels (A) and leaves through one efferent vessel (E) at the hillus. During the passage of lymph through the node there is removal of particulate antigens by the phagocytic cells and then this is transported to the lymphoid region of the node. The cortex is a B cell area and the paracortex is a T cell area. Afferent lymph vessel Cortex Afferent lymph vessel Paracortex Efferent lymph vessel Germinal Centre Blood Vessel Afferent lymph vessel primary follicle germinal center secondary follicle Mucosal Associated Lymphoid Tissue (MALT) are aggregates of lymphoid tissue which do not have a tough outer capsule. They are found especially in the lamina propria, and sub-mucosal areas of the gastrointestinal, respiratory and genito-urinary tracts. Typical examples of MALT are tonsils and appendix. Other examples include Peyer’s patches (right). These are organised regions of lymphoid tissue found in the wall of the gut. Gut wall Lumen B cell area T cell areas Page 3 of 11 2) Outline the recirculation of lymphocytes. Small B and T lymphocytes which have matured in the bone marrow or thymus and which have not yet encountered antigen are called naïve lymphocytes. These cells circulate constantly from the blood into the secondary lymphoid organ and leave the vasculature through a specialised section of the post capillary venule known as the high endothelial venule (HEV). They move from the lymph node to the lymphoid vessels and eventually return to the blood via the thoracic duct. In the presence of an infection those cells which recognise the infectious agent are held in the lymphoid tissue where they proliferate and differentiate. Recirculation Virgin Lymphocytes Blood Peripheral Lymphoid tissue Activated Lymphocytes Death 3) Explain the use of CD (cluster of differentiation) markers for distinguishing different immune cell types. Cluster of differentiation - an internationally recognized systematic nomenclature for cell surface molecules used to discriminate between cells of the hematopoietic system. Different lineages or maturational stages of lymphocytes can be distinguished by their expression of membrane molecules recognised by particular monoclonal antibodies. All of the monoclonal antibodies that react with a particular membrane molecule are grouped together as a cluster of differentiation. Each new monoclonal antibody that recognises a leukocyte membrane molecule is analysed for whether it falls within a recognised CD designation; if it does not, it is given a new CD designation reflecting a new membrane molecule. T Lymphocytes In B cells, CD 19 and CD20 is expressed at the surface T cells all express CD3 + TCR, about 10%in blood + TCR, about 90%in blood ~2/3 express CD4 ~1/3 express CD8 4) Compare and contrast the phenotypic characteristics of T and B lymphocytes and T lymphocyte subsets. T cells B cells Recognise antigen presented at the cell surface by MHC Recognise free antigen in the body fluids molecules Antigen receptor is surface Ig like molecule Antigen receptor is either or TCR. All T cells express CD3. Most T cells express either CD4 or CD8 Express CD19 & CD20 at surface Produced by Thymus Produced by Bone Marrow 5) Give examples of professional antigen presenting cells (APCs) and state their locations. Cells that can present antigen to lymphocytes in an immunogenic form are collectively termed antigen presenting cells or APCs. Cell Location Presents to Langerhans Cells (=Dendritic cells) Widely spread e.g. Skin & mucosal tissue T cells Follicular Dendritic Cells Follicles B cells B cells Lymphoid Tissue T cells Activated Macrophages Lymphoid Tissue T cells Lecture 3: Innate immunity 1) Briefly describe the functions of the important phagocytic cells: neutrophils, monocytes/macrophages. Neutrophils: 50-70% of leukocytes; short-lived cells (1-3 days), circulate in blood then migrate to tissues; first cells to arrive at a site of tissue damage/infection. (defunct neutrophils are a major constituent of pus). Neutrophils enclose material in a phagosome, the phagosome fuses with both primary and secondary granules and its contents are degraded by two different pathways: oxygen dependent degradation –invoving reactive oxygen, hydrogen peroxide and HOCl acid; oxygen-independent degradation this is carried out by lysosomes and hydrolytic enzymes. Neutrophils act by firstly adhering to the epithelium (rolling and signal from endothelium cause adherence), then movement into tissue-diapediesis, the neutrophils then binds to the pathogen (homed in by chemotactic gradient), phagocytosis then occurs (which includes opsonisation- coating of microorganisms with proteins to facilitate phagocytosis- opsonin binds to antigen and cqn also be bound by phagocytes. Antibodies and complemtent also act as opsonins). This is followed by killing, which is by oxygen dependent or independent actions as mentioned before. Macrophages: less abundant account for about 5-10% of leukocytes, dispersed throughout the tissues (they circulate for about 8hrs before they enter the tissues);in the tissues they differentiate into macrophages; macrophages are larger and longer lived than monocytes and have greater phagocytic ability (as well as having a larger repertoire of lytic enzymes); signal infection by Page 4 of 11 2) 3) release of soluble mediators (cytokines). In addition to phagocytosis macrophages are able to process and present antigen in association with class II MHC (i.e. act as APCs) Killing mechanisms are (like neutrophils): oxygen-independent (e.g. enzymes and microbial peptides) or oxygen-dependent (e.g. respiratory burst and production of toxic metabolites/reactive nitrogen intermediates). In comparison to neutrophils, macrophages are: Longer lived Larger- enabling phagocytosis of larger targets Move more slowly Exhibit less pronounced respiratory burst Phagocytose more slowly Retain glogi and RER for secretions Act as APCs Define cytokines and describe their general properties. Cytokine – any of numerous secreted, low-molecular-weight proteins that regulate the intensity and duration of the immune response by exerting a variety of effects on lymphocytes and other immune cells. They stimulate or inhibit the activation, proliferation, and/or differentiation of various cells and regulate the secretion of antibodies or other cytokines. The complex cellular interactions involving cells of the immune, inflammatory, and haematopoietic systems are mediated by cytokines. Most cytokines act on nearby target cells (paracrine action), although in some cases a cytokine can act on the cell that secretes it (autocrine action) or on a distant cell (endocrine action). Define complement, list its major functions, and draw a simple diagram of the complement pathways. Complement – a group of serum proteins that participates in an enzymatic cascade, ultimately generating the cytolytic membraneattack complex. It components are mainly produced by the liver and monocytes/macrophages. Its major functions are: 1. Lysis of cells, bacteria, and viruses. 2. Opsonisation, which promotes phagocytosis of particulate antigens. 3. Binding to specific complement receptors on cells of the immune system, triggering activation of immune responses such as inflammation and secretion of immunoregulatory molecules that amplify or alter specific immune responses. 4. Immune clearance, which removes immune complexes from the circulation and deposits them in the spleen and liver. There are 3 pathways: 1. The classical pathway – initiated by antigen –antibody complexes 2. The alternative pathway - continuous tickover production, with full activation upon fixation to pathogen surface 3. The lectin pathway – antibody independent activation of the classical pathway The classical and alternative pathways converge at C3. A meeting point for the adaptive and innate immune systems. C3 leads to to a final common pathway – late phase of complement activation, and ends in the formation of the ‘Membrane Attack Complex’ (MAC) 4) Describe a typical inflammatory response to a localised infection involving recruitment of neutrophils, and phagocytosis and killing of bacteria. 1. Vasodilation - tissue redness, rise in tissue temperature 2. Increase in vascular permeability (particularly postcapillary venules) – oedema (tissue swelling due to accumulation of fluid). Complement anaphylatoxins induce mast-cell degranulation with release of histamine causing vasodilation and increased vascular permeability. 3. Influx of phagocytes from capillaries (margination, diapedesis and finally chemotaxis). Neutrophils phagocytose invading pathogens and release chemokines that attract macrophages to the site of inflammation. Macrophages are activated cells that exhibit increased phagocytosis and increased release of mediators and cytokines that contribute to the inflammatory response. Release cytokines that induce expression of adhesion molecules on vascular endothelial cells. Thus circulating neutrophils, monocytes and lymphocytes adhere to the wall of a blood vessel by recognising these adhesion molecules and then move through the vessel wall into the tissue spaces. 5) Briefly outline the events involved in a systemic acute phase response. A local inflammatory respose may be accompanied by a systemic response ‘acute phase’ after 1-2 days Marked by the induction of fever, increased hormone synthesis, increased production of white blood cells (leukocytosis) and production of a large number of acute-phase proteins in the liver. Increase in body temperature inhibits the growth of a number of pathogens and appears to enhance the immune response to the pathogen. Page 5 of 11 C-reactive protein (acute-phase protein) levels increase 1000-fold. Binds to a wide variety of microorganisms and activates complement resulting in opsonisation and phagocytosis. Cytokines (IL-1, TNF-α, IL-6) act on the hypothalamus to induce a fever response. Colony stimulated factors stimulate haematopoiesis, resulting in transient increases in the number of white blood cells needed to fight the infection. 6) Outline the phenotype and functions of natural killer (NK) cells. Natural killer cells – a class of large granular cytotoxic lymphocytes that do not have T- or B-cell receptors. They are antibody independent killers of tumour cells and viral cells and also can participate in antibody-dependent cell-mediated cytotoxicity. Recruited to site of infection by chemokines. They also secret interferons. NK cells make up 5-10% of the peripheral blood lymphocytes - Lecture 4: Antibodies 1) Describe with the aid of a simple diagram the immunoglobulin molecule, identifying the antigen-binding site (Fab) and Fc portions of the molecule. An antibody is a protein that is produced in response to a foreign molecule (antigen) and has the property of binding specifically to the antigen that induced its formation. Antibodies constitute the class of proteins known as immunoglobulins. 2) Briefly describe the properties of the antigenbinding site. Light chains contain one variable and one constant domain, the heavy chain contains one variable and three constant domains Each variable region has three hypervariable regions. The hypervariablity regions constitute the antigen-binding site and therefore antigen specificity of a particular antibody. Because they are complementary to the structure of the eptitope they bind. The hypervariability regions are also known as the complementary determining regions (CDRs). About 6 amino acids/ sugar units will fit into the binding sites, the nature of the binding includes hydrophobic, ionic and H-bonding The wide range of specificities exhibited by antibodies is due to variations in the length and amino acid sequence of the six hypervariable loops in each Fab fragment. 3) Distinguish between antibody affinity and antibody avidity. Affinity is a measure of the strength of binding between a single binding site of an antibody and its antigen. Avidity is the overall strength of binding between antibody and antigen, taking into account the number of binding sites on the antibody and the number of site on the antigen (antigenic determinants or epitopes) that can be bound. 4) List the immunoglobulin classes and subclasses in man. Describe their functions and relate these to their individual structure. IgG This is the most abundant Ig in blood and tissue spaces and is the most important in defence generally - the other Ig classes have a more specialised role. IgG neutralises toxins and viruses. Further, it performs a number of functions that are dependent on the nature of its Fc. When IgG binds to a bacterium or other infectious agent, macrophages or neutrophils recognise the Fc (they possess Fcγ receptors) and this leads to phagocytosis (opsonisation). In the same situation complement activation can take place promoting phagocytosis or lysis of the bacterium. IgG crosses the placenta and, because of its comparatively long half-life, is able to provide protection in the newborn child for at least 3 months. (see above for diagram of structure). There are 4 sub-classes of IgG: IgG1 (70% of total IgG), IgG2 (20%), IgG3 (7%), IgG4 (3%). For most of these functions, IgG1 and 3 are more active than IgG2 and 4. Antibodies to bacterial carbohydrates are often IgG2. IgA IgA is present in the blood where it occurs in monomer form, but its function there is not understood. IgA has an important role in seromucous secretions where it occurs as secretory IgA - a dimer together with J chain and secretory component (SC). SC is added to the molecule during its passage through epithelial cells out into the secretions, and it helps to protect the molecule from degradative enzymes that may be present there (e.g., in the gut). Secretory IgA can neutralise toxins. Moreover, by binding to infectious agents it can block their infectivity - often by preventing adherence of the agents to the epithelial cells. There are two sub-classes of IgA: IgA1 and IgA2. While IgA1 predominates in the blood, IgA2 is strongly represented in the secretions. Page 6 of 11 IgM is the first Ig to be made following contact with an antigen, and it is active in the blood. Its five Fab pairs allow it to bind strongly to the surface of bacteria causing agglutination. Like IgG it is adept at activating complement. IgE Mast cells and basophils have a high affinity receptor for the Fc of IgE(FcεR1). When IgE that is bound in this way is crosslinked by antigen, degranulation of the cells occurs with release of inflammatory mediators such as histamine. This process is important in protection against certain parasitic infections, but it can be a nuisance giving rise to allergic diseases such as hay fever or life-threatening anaphylaxis. Lecture 5: B lymphocytes 1) Briefly outline the principles of immunoglobulin gene rearrangement in the generation of diversity. The process of Ig gene rearrangement ensures that when any foreign antigen enters the body there will be a few B lymphocytes with Ig molecules on their surface able to bind to that antigen. After binding to the antigen, such a lymphocyte will divide to form a clone of identical cells with the capacity to produce antibody of this specificity. Remember that each B lymphocyte in the body has the capacity to make antibody of only one specificity. The antigen thus selects for a clone which has the capacity to produce antibody able to bind that antigen. An important aspect of the process of B cell stimulation is to produce antibody-secreting plasma cells and memory cells in the cooperation with T cells. Genomic rearrangement is an essential feature of lymphocyte differentiation, and no other vertebrate cell type has been shown to undergo this process. Three chromosomes are involved in coding for Ig chains. One is responsible for all the κ (kappa) light chains, one for λ (lamda) light chains and one for all the heavy chains. The principles for rearrangement are the same for all three. The genes coding for the variable regions are stored together up-stream of the genes for the constant region. In this way it is not necessary to repeat the constant region DNA for each different variable region. To make matters more complicated the variable genes exist in segmented form before rearrangement. That is to say for kappa light chains one has a series of V segments which code for most of the variable region followed by a series of J segments which code for the rest of the variable region. During re-arrangement, which will convert a progenitor B cell into a functional B cell, one of the V segments comes together with one of the J segments. Unwanted DNA is looped out by a special mechanism. It is then possible to produce a primary RNA consisting of VJ linked to the constant region(C). Unwanted RNA between J and C is spliced out to give mRNA for VJC. This can then be translated into the light chain. The process is similar for lamda light chains and the heavy chains, but for the heavy chains there is a series of D segments between the V and J segments and the mRNA thus represents VDJC. Diversity is further extended by variation in the precise joining points of the V, D and J segments, somatic mutation of the rearranged genes and the random pairing of H and L chains to make the Ig molecule. The number of different antibody specificities that can be produced by these processes is enormous. The heavy chain constant genes are sited one after the other, starting with Cμ, following the variable region genes. Under the influence of T cells, a process of class switching can occur in which the already re-arranged VDJ can switch its attachment from Cμ to Cγ, Cα or Cε. This produces the different classes of antibody but does not affect antibody specificity. 2) Describe the process of stimulation of individual B cells to divide and secrete antibody such as to generation immunity to a particular antigen (clonal selection). The process of Ig gene rearrangement (see above) ensures that when any foreign antigen enters the body there will be a few B lymphocytes with Ig molecules on their surface able to bind that antigen. After binding the antigen, such a lymphocyte will divide to form a clone of identical cells with the capacity to produce antibody of this specificity. Remember that each B lymphocyte in the body has the capacity to make antibody of only one specificity. The antigen thus 'selects' a clone which has the capacity to produce antibody able to bind that antigen. 3) Differentiate between monoclonal and polyclonal antibodies. When an antigen enters the body the antibody response that occurs is normally polyclonal. That is to say a heterogeneous mixture of antibodies are produced. There will be antibodies directed to several different determinants on the antigen, and the antibodies to a particular determinant will be of variable affinity. So these are polyclonal antibodies: they derive from many different clones of B cells. Monoclonal antibodies are homogeneous being derived from a single B cell clone. They are normally made artificially by fusing one antibody-producing cell, making a particular molecule of antibody, with a tumour cell to form a clone of cells which will divide and produce this antibody for a long time. Monoclonal antibodies are useful reagents for detecting cell markers such as CD4 or CD8. Myeloma proteins are the monoclonal Ig products of a plasma cell tumour. Such tumours may arise spontaneously causing major clinical problems. They are helpful in studying Ig structure, but the antigen to which they bind is generally unknown. Page 7 of 11 4) Outline the differences in antibody production during primary and secondary immune responses. Memory cells show qualitatively different and quantitatively enhanced responses upon re-exposure. A second encounter with the same antigen induces a heightened state of immune reactivity. A secondary response comes much more quickly, it is more intense and longer lasting, also IgG is the major player as opposed to IgM, which was the main type of antibody in the primary response. Lecture 6: T lymphocytes 1) Outline the origins and functions of T lymphocyte subsets. T cell maturation occurs in the thymus as progenitor cells from the bone marrow enter the thymus and rearrange their TCR genes. The earliest thymocytes lack detectable CD4 and CD8 and are referred to as double-negative cells. These double-negative thymocytes differentiate along two developmental pathways. Those thymocytes that make a productive rearrangement of the γδ TCR genes develop into CD4-, CD8-, CD3+ γδ T cells, which account for only a small minority of thymocytes. The majority of double-negative thymocytes rearrange the αβ TCR genes and develop into CD4+, CD3+ αβ T cells or CD8+, CD3+ αβ T cells. Immature thymocytes in the αβ pathway express a pre-T-cell receptor. CD8+ cells (Tc or cytotoxic)are cytotoxic and kill target cells- they secrete cytokines and induce apoptosis (cell death) in the target cell. Schematic representation of T cell development THYMUS Bone marrow CD4+ CD8- Cortex Medulla CD4CD8- 2) CD4+ CD8+ Naïve T cells CD4CD8+ CD4+ cells (T helper or Th cells) secrete cytokines – which recruit effector cells of innate immunity, also amplify and help Tc and B cell responses. Briefly describe the structure and distribution of major histocompatibility complex (MHC) class I and class II molecules. T cells recognise antigen only when it is associated with a molecule of major histocompatability complex (MHC) T cells recognise peptide fragments of an antigen in association with MHC molecules rather than its individual cornformation (as with B cells). Therefore an antigen needs to be processed before it can presented to a T cell. T cell antigen receptor consists of the T cell receptor (TCR) associated with CD3 (the cluster of differentiation that all T cells contain). The TCR recognises and binds antigen and CD3 is involved in signal transduction. The MHC is a group of tightly linked genes important in specific immune responses, in humans the MHC is also called the human leucocyte antigen (HLA). MHC molecule present antigens to T lymphocytes. T-cell receptors can recognise only antigen that is bound to major histocompatibility complex (MHC) molecules. MHC molecules that function in this recognition event (antigen presentation) are polymorphic glycoproteins found on all cell membranes. The MHC exhibit a broad specificity in binding of peptide. The polymorphism of the MHC is largely concentrated in the peptide-binding cleft There are two major types of MHC molecules: MHC class I molecules, which are expressed by nearly all nucleated cells and present endogenous antigen to CD8+ cells, consist of a heavy chain linked to a small invariant protein called β2-microglobulin. Class II MHC molecules, which consist of an α and a β glycoprotein chain, are expressed only by “professional” antigen-presenting cells (macrophages, dendritic cells and B lymphocytes). These present exogenous antigen to CD4+ T cells ~90aa S S N ~90a a MHC CLASS I PEPTIDE BINDING REGION N S S S S ~90aa IMMUNOGLOBULIN LIKE REGION ~90a a -microglobulin C TRANSMEMBRANE REGION ~25aa CYTOPLASMIC REGION ~30aa C ~90aa N NS S S S S S ~25aa variable length MHC CLASS II ~90a PEPTIDE BINDING a REGION ~90a IMMUNOGLOBULIN LIKE REGION a TRANSMEMBRANE REGION C C CYTOPLASMIC REGION 3) Outline the mechanisms by which antigen presenting cells (APCs) process and present endogenous and exogenous antigens. In order for a foreign protein antigen to be recognised by a T cell, it must be degraded into small antigenic peptides that form complexes with class I or class II molecules. This conversion of proteins into MHC-associated peptide fragments is called antigen processing and presentation. Whether a particular antigen will be processed and presented with class I MHC or class II MHC molecules appears to be determined by the route that the antigen takes to enter the cell. Page 8 of 11 Exogenous antigen (extracellular) is produced outside of the host cell. Antigen presenting cells degrade ingested exogenous antigen into peptide fragments within the endocytic pathway (in lysosomal compartments) and peptides produced bind to the cleft within the class II MHC molecules and the complex is then exported to the surface. Since expression of class II MHC complexes is limited to APCs, presentation of exogenous peptide-class II MHC complexes is limited to these cells. T cells displaying CD4 recognise antigen combined with MHC class II molecules and are said to be class II MHC restricted. Generally function as T helper cells. Endogenous antigen (cytoplasmic) is produced within the host itself (e.g. viral proteins and proteins synthesised by cancerous cells). Endogenous antigens are degraded into peptide fragments that bind to class I MHC molecules within the endoplasmic reticulum. The peptide-class I MHC complex is then transported to the cell membrane. Since all nucleated cells express class I MHC molecules, all cells producing endogenous antigen use this route to process the antigen. T cell displaying CD8 recognise antigen associated with class I MHC molecules and thus are said to be class I MHC restricted. These T cytotoxic cells attack and kill cells displaying the antigen-MHC class I complexes for which their receptors are specific. 4) Compare and contrast antigen recognition by B and T lymphocytes and by CD4+ and CD8+ T lymphocytes. The cell membranes of both B and T lymphocytes have antigen-binding receptors. The receptors on B cells are antibody molecules, which can recognise and interact directly with antigen. T-cell receptors, in contrast, recognise only antigen that is combined with either class I or class II MHC molecules on the surface of a cell. As B and T lymphocytes mature, each cell comes to express receptors that recognise a single antigenic determinant (epitope). In general, TH cells express CD4 and recognise combined with class II MHC cells, whereas TC cells express CD8 and recognise antigen combined with class I MHC cells. Lecture 7: Adaptive immune response 1) Outline the importance of antigen presenting cells in the induction of T lymphocyte responses Naïve T cells do not have effector functions- they need to be activated by exposure to an antigen However the antigen must be processed and presented by an antigen presenting cell (APC) – these include dendritic cells, B cells, follicular cells and macrophages 2) Describe the effector functions of T lymphocytes including cell-mediated cytotoxicity, macrophage activation, delayed hypersensitivity and T/B lymphocyte co-operation. CD8+ effector T cells kill target cells that present peptides of cytosolic pathogens (viruses) in context with MHC class I molecules on their cell surface. Cytotoxic T cells (CTLs) kill their target cells by programmed death- apoptosis, which is characterised by fragmentation of nuclear DNA. Effector CTLs secrete granules, which form pores in the cell membrane and allow other granules in to cause fragmentation. CD4+ Th1 effector cells activate infected macrophages which present peptides in context with MHC class II molecules on their cell surface. Th1 effectors produce IFN-γ, IL-2, TNF-β. CD4+ Th2 effector cells are also MHC class II restricted and help B cells to differentiate into antibody secreting plasma cells. Th2 effector cells produce IL-4, IL-5, IL-6, IL-10, IL-13. Effector cells differ from naïve T cells Two signals, antigen-specific recognition (TCR-antigen) and a co-stimulatory signal (CD28-B7) are required for activation of naïve T cells. Effector T cells do not require the co-stimulatory molecule to act. Cell mediated cytotoxicity Cytotoxic T cells (CTL) kill their targets by programmed cell death i.e. apoptosis which is characterised by fragmentation of nuclear DNA. CTLs store perforin and granzymes in their granules. After recognition of infected targets, granules are released, perforin molecules polymerise to form pores in the target membrane. Fas-FasL interaction is also involved in the killing. Macrophage activation: Inflammatory Th1 effector cells activate macrophages to promote killing of intracellular pathogens (mycobacteria, leishmania, etc.). Activated macrophages express increased levels of CD40 and TNF-α receptors, and secrete TNF-α which synergises with IFN-γ in the induction of antimicrobial effector mechanisms. Delayed type hypersensitivity (DTH) reaction: Mediated by pre-existing antigen-specific T cells, mainly by inflammatory Th1 cells, CD4+ Th1 cells release inflammatory cytokines that affect blood vessels (TNF-β), recruit (chemokines) and activate (IFN-γ) macrophages. DTH inducers: intracellular parasites (Leishmania), intracellular bacteria (Mycobacteria), intracellular fungi (Candida), intracellular viruses (Herpes simplex). Local swelling with cellular infiltrates occur 24-72 hours after antigen exposure. Page 9 of 11 T-B cell collaboration: Immunoglobulin (Ig)+ B cells bind specific antigen. The Ig-antigen complex is internalised, processed and antigenic peptides are presented on the B cell surface in context with MHC class II molecules. T helper cells with specific TCR recognise antigen-MHC complex on the cell surface. The T-B interactions trigger expression of CD40 ligand (CD40L) on T cells. CD40L will interact with CD40 expressed by B cells T cells secrete cytokines and B cells express cytokines receptors. The activated B cell will differentiate into immunoglobulin (antibody) secreting plasma cells. 3) Briefly outline the function of T helper cells in relation to the different cytokines they produce. Th1 cells and their soluble effector molecules (IFN-γ, IL-2, TNF-β) are involved in cell-mediated immunity. Th2 cells and their soluble effector molecules (IL-4, IL-5, IL-6, IL-10, IL-13) function in humoral immunity. Th1 associated functions: Cytokines involved Macrophage activation IFN-γ, TNF-α Delayed type hypersensitivity reaction IL-2, IFN-γ, TNF-α, IL-3, GM-CSF Help for CD8 cells IL-2 Downregulation of Th2 responses IFN-γ Th2 associated functions: Cytokines involved B cell proliferation IL-2, IL-4, IL-5 B cell differentiation and immunoglobulin class switch IL-2, IL-4, IL-5, IFN-γ, TGF-β Downregulation of Th1 responses IL-4, TGF-β, IL-10 4) Explain the different requirements for activation of naïve and memory T lymphocytes Naïve cells: 2 signals, antigen-specific recognition (TCR antigen) and co-stimulatory signal (CD28-B7) are required to activate naïve T cells Whereas memory effector cells do not require the co-stimulatory molecule to react Lecture 8: Host defence overview 1) Define and contrast innate and adaptive/acquired immunity, humoral and cellular immunity. Name the important components of each. Immunity – the state of protection from infectious disease – has both non-specific and specific components. The non-specific component, innate immunity is a set of disease-resistant mechanisms that are not specific to a particular organism. Phagocytic cells, such as macrophages play an important role in many aspects of innate immunity. Innate immunity can be seen to comprise four types of defensive barriers: anatomic (e.g. skin and mucous membranes), physiologic (e.g. temperature, low pH and chemical mediators), phagocytic, and inflammatory. In contrast, the specific component, adaptive immunity, exhibits four immunological attributes: specificity, diversity, memory, and self/non-self recognition. Functionally, an immune response consists of two interrelated events: recognition of antigen and response to that antigen (i.e., generation of effector cells and molecules). Antigen-presenting cells, B lymphocytes, and T lymphocytes are the primary cells of the immune response. The humoral branch of the immune system is at work in the interaction of B cells with antigen and their subsequent proliferation and differentiation into antibody-secreting cells. Antibody functions as the effector of the humoral response by binding to antigen and neutralizing it or facilitating its elimination. Effector T cells generated in an immune response to antigen are responsible for cell-mediated immunity. Both activated TH (T helper) cells and CTLs (cytotoxic T lymphocytes) serve as effector cells in cell-mediated immune reactions. Cytokines secreted by TH cells can activate various phagocytic cells, enabling them to phagocytose and kill microorganisms more effectively. CTLs participate in cell-mediated immune reactions by killing altered self-cells; they play an important role in the killing of virus-infected cells and tumour cells. 2) Summarise and give examples of the roles taken in host defence mechanisms by: physical or chemical boundaries; mechanical removal; colonisation resistance; nonspecific immune responses. Anatomic barriers Epidermis of the skin. Sebaceous glands in dermis of skin secrete sebum containing lactic and fatty acids which maintain the pH of the skin between 3 and 5, thus inhibiting the growth of most microorganisms. Mucous membranes – saliva, tears and mucous secretions act to wash away potential invaders and also contain antibacterial and antiviral substances. Mucous entraps foreign microorganisms. Cilia propels mucus-entrapped microorganisms from the respiratory and gastrointestinal tract. Nonpathogenic organisms tend to colonize the epithelial cells of mucosal surfaces, outcompeting pathogens for attachment sites and necessary nutrients (commensals) Physiological barriers Temperature – high temperature inhibits growth of pathogens. pH – few ingested microorganisms can survive the low pH of the stomach contents. Lysozyme – hydrolytic enzyme found in mucous secretions and tears; able to cleave the peptidoglycan layer of the bacterial cell wall. Interferon – group of proteins produced by virus-infected cells; ability to bind to nearby cells and induce a generalised antiviral state. Complement – group of serum proteins that circulate in an inactive state. Active forms damage the membranes of pathogenic organisms, either destroying the organisms or facilitating their clearance. Phagocytic barriers: Most phagocytosis is conducted by specialised cells, such as blood monocytes, neutrophils, and tissue macrophages. Inflammatory barriers: Tissue damage caused by a wound or by and invading pathogenic microorganism induces a complex sequence of events collectively known as the inflammatory response. 1. Vasodilation - tissue redness, rise in tissue temperature Page 10 of 11 Increase in capillary permeability – oedema (tissue swelling due to accumulation of exudate) Influx of phagocytes from capillaries (margination, diapedesis and finally chemotaxis). The accumulation of dead cells, digested material, and fluid forms pus. 3) Appreciate that immune reactions at mucosal sites are treated in a different way to those encountered elsewhere in the body. Mucosa is lining the surfaces of the respiratory, gastrointestinal and urogential tracts as well as the eye conjunctiva. Immune responses at mucosal surfaces must be appropriate. A fine balance exists between reacting to an antigen and ignoring or being tolerant to it. Antigens in the periphery elicit an immune response. Antigens at mucosal surfaces are either ignored or encounter mechanisms that try to physically exclude them. IgA is essential at mucosal surfaces because it complexes the antigen in the lumen of the gut or lung. This leads to physical exclusion of the antigen from the body interior. Mucosal diseases occur when tolerance breaks down. For examples, a disregulation of immune responses in the gut is responsible for cow milk enteropathy, celiac disease, necrotising enterocolitis and reactivity to normal gut flora. 4) List defence mechanisms operative against major pathogens. Defences against bacteria Surface defences (mechanical and chemical) Antibody opsonisation Activation of complement (alternative pathway) causing lysis/opsonisation (coating of microorganisms with protein to facilitate phagocytosis) Phagocytosis Trigger release of soluble inflammatory mediators and acute phase proteins by host cells (especially phagocytes) causing fever, opsonisation etc. Defences against viruses Surface defences (mechanical and chemical) NK cells Interferons Antibody opsonisation (partially effective) Trigger release of soluble inflammatory mediators and acute phase proteins by host cells (especially phagocytes) causing fever, opsonisation etc. T cells 5) Describe two attributes of an immunological memory response. Immunological memory is one of the hallmarks of adaptive immune responses. Memory responses are faster and greater in magnitude than primary immune responses. Immunological memory can confer life-long protection to many infections, is basis of vaccination. Memory cells show qualitatively different and quantitatively enhanced responses upon re-exposure. 2. 3. Page 11 of 11