lecture 6 Dr Numan Nafie Hameed د. نعمان نافع الحمداني Perinatal
advertisement

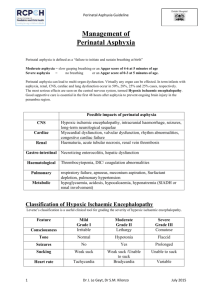
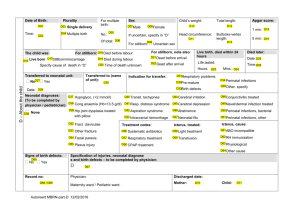
lecture 6 Dr Numan Nafie Hameed نعمان نافع الحمداني.د Perinatal asphyxia Definition. Perinatal asphyxia is a condition caused by a lack of oxygen in respired air, resulting in impending or actual cessation of apparent life (Clinical). Perinatal asphyxia is a condition of impaired blood gas exchange that, if it persists, leads to progressive hypoxemia and hypercapnia with a metabolic acidosis.(Biochemical) Essential characteristics defined jointly by the American Academy of Pediatrics (AAP) and the American College of Obstetricians and Gynecologists (ACOG) should be present: (1) profound metabolic or mixed acidemia (pH 7.00) on umbilical cord arterial blood sample, if obtained; (2) persistence of an Apgar score of 0-3 for 5 min; (3) neurologic manifestations in the immediate neonatal period to include seizures, hypotonia, coma, or hypoxic-ischemic encephalopathy (HIE); and (4) evidence of multiorgan system dysfunction in the immediate neonatal period. Biochemical indices. There is no specific blood test to diagnose perinatal asphyxia. 1. The normal umbilical arterial base excess is - 6 mEq/L with -10 to -12 mEq/L as the upper statistical limit of normal. Base excess -20 mEq/L is required to show neurologic damage associated with metabolic acidosis. 2. The precise value that is required to define damaging acidemia is not known. A pH 7.0 realistically represents clinically significant acidosis. Acidemia alone does not establish that hypoxic injury has occurred. Apgar score 1. Conceived to report on the state of the newborn and effectiveness of resuscitation. It is a poor tool for assessing asphyxia. Low Apgar scores are unlikely to be the cause of morbidity but rather the results of prior causes. 2. An infant with an Apgar score of 0-3 at 5 min, improving to 4 by 10 min, has 99% chance of not having cerebral palsy (CP) at 7 years of age; 75% of children who develop CP have normal Apgar scores at birth. 3. A 1996 revised AAP/ACOG statement again emphasized that the Apgar score alone should not be used as evidence that neurologic damage was caused by hypoxia resulting in neurologic injury or by inappropriate intrapartum management. 1 Mechanisms of asphyxia during labor, delivery, and the immediate postpartum period.(Causes): A. Interruption of the umbilical circulation (cord compression). B. Inadequate perfusion of the maternal side of the placenta (maternal hypotension, hypertension, abnormal uterine contractions). C. Impaired maternal oxygenation (cardiopulmonary disease, anemia). D. Altered placental gas exchange (placental abruption, previa, insufficiency). E. Failure of the neonate to accomplish lung inflation and successful transition from fetal to neonatal cardiopulmonary circulation. Pathophysiology A. Adaptive responses of the fetus or newborn to asphyxia. The fetus and neonate are much more resistant to asphyxia than adults. In response to asphyxia, the mature fetus redistributes the blood flow to the heart, brain, and adrenals to ensure adequate oxygen and substrate delivery to these vital organs. B. Impairment of cerebrovascular autoregulation results from direct cellular injury and cellular necrosis from prolonged acidosis and hypercarbia. C. The majority of neuronal disintegration occurs after termination of the asphyxial insult because of persistence of abnormal energy metabolism and low (ATP) levels. A cascade of deleterious events is triggered, resulting in formation of free radicals, increased extracellular glutamate, increased cytosolic Ca2+, and delayed cell death. D. Major circulatory changes during asphyxia 1. Loss of cerebrovascular autoregulation under conditions of hypercapnia, hypoxemia, or acidosis. Cerebral blood flow (CBF) becomes "pressure passive," leaving the infant at risk for cerebral ischemia with systemic hypotension and cerebral hemorrhage with systemic hypertension. 2. Increase in CBF secondary to redistribution of cardiac output, initial systemic hypertension, loss of cerebrovascular autoregulation, and local accumulation of vasodilator factors (H+, K+, adenosine, and prostaglandins). 3. With prolonged asphyxia, there is a decrease in cardiac output, hypotension, and a corresponding fall in CBF. In general, brain injury occurs only when the asphyxia is severe enough to impair CBF. E. The postasphyxial human newborn is in a persistent state of vasoparalysis and cerebral hyperemia, the severity of which is correlated with the severity of the asphyxial insult. 2 Cerebrovascular hemorrhage may occur on reperfusion of the ischemic areas of the brain. However, when there has been prolonged and severe asphyxia, local tissue recirculation may not be restored because of collapsed capillaries in the presence of severe cytotoxic edema. F. Cerebral edema is a consequence of extensive cerebral necrosis rather than a cause of ischemic cerebral injury. Neuropathologic findings A. Cortical changes. Cortical edema, with flattening of cerebral convolutions, is followed by cortical necrosis until finally a healing phase results in gradual cortical atrophy. Cortical atrophy, if severe, may result in microcephaly. B. Selective neuronal necrosis is the most common type of injury observed in neonatal HIE. C. Other findings seen in term infants include status marmoratus of the basal ganglia and thalamus (the marbled appearance is a result of the characteristic feature of hypermyelinization) and parasagittal cerebral injury. D. Periventricular leukomalacia (PVL) is hypoxic-ischemic necrosis of periventricular white matter resulting from cerebral hypoperfusion. Injury to the periventricular white matter is the most significant problem contributing to long-term neurologic deficit in the premature infant, although it does occur in sick full-term infants as well. The incidence of PVL increases with the length of survival and the severity of postnatal cardiorespiratory disturbances. PVL involving the pyramidal tracts usually results in spastic diplegic or quadriplegic CP. E. Porencephaly, hydrocephalus, hydranencephaly, and multicystic encephalomalacia may follow focal and multifocal ischemic cortical necrosis, PVL, or intraparenchymal hemorrhage. F. Brainstem damage is seen in the most severe cases of hypoxic-ischemic brain injury and results in permanent respiratory impairment. Clinical presentation A. The majority of infants who experience intrauterine hypoxic-ischemic insults do not exhibit overt neonatal neurologic features or subsequent neurologic evidence of brain injury. It is generally accepted that after acute perinatal asphyxia there should be an acute encephalopathy, often accompanied by multiorgan malfunction. B. Occurrence of neonatal neurologic syndrome The primary signs of CNS injury in the term infant include seizures, apnea, posturing and movement disorders, impaired suck, and jitteriness. The absence of this neonatal neurologic syndrome rules out intrapartum insult as the cause of major brain injury. 3 C. The severity of HIE correlates with the duration and severity of the asphyxial insult. A constellation of neurologic signs evolves over the first 72 h of life best characterized by Sarnat and Sarnat in 1976: stage I (hyperalert, awake state), stage 2 (lethargic, obtunded, hypotonic, seizures), stage 3 (stuporous, comatose, flaccid, posturing). Moderately to severely affected infants are usually obtunded if not comatose, with generalized hypotonia and paucity of spontaneous movements. Depressed reflexes and cranial nerve palsies are common findings. Presentation of hypertonicity and irritability generally are not noted until the second week of life. D. Occurrence of seizures within the first 12-24 h after birth is indicative of intrapartum insult until proven otherwise. Seizures may also be secondary to hypoglycemia. E. Hypoxic-ischemic spinal cord injury. Ischemic injury to anterior horn cells within the spinal cord gray matter is relatively common among hypotonic and hyporeflexic neonates after severe perinatal hypoxia-ischemia. Electromyographic examinations show injury to the lower motor neuron above the level of the dorsal root ganglion . F. Clinical presentation may be further obscured by the coexistence of skull fracture, subdural hematoma, or subarachnoid hemorrhage resulting from traumatic delivery. G. Multiple organ involvement. involvement of 1 or more organs occurred in 82% of infants with perinatal asphyxia. The central nervous system (CNS) is the organ most frequently involved . 1. Cardiovascular system. Shock, hypotension, tricuspid insufficiency, myocardial necrosis, congestive heart failure, and ventricular dysfunction. 2. Renal function. Oliguria-anuria, acute tubular or cortical necrosis (hematuria, proteinuria), and renal failure. 3. Hepatic function. Elevated serum -glutamyl transpeptidase activity, ammonia and indirect bilirubin, and decreased clotting factors at 3-4 days' postnatal age in moderate to severe asphyxia. 4. Gastrointestinal tract. Paralytic ileus or delayed (5-7 days) necrotizing enterocolitis. 5. Lungs. Respiratory distress syndrome from surfactant deficiency or dysfunction, pulmonary hemorrhage (shock lung), and persistent pulmonary hypertension. 6. Hematologic system. Thrombocytopenia can result from shortened platelet survival or DIC. Increased numbers of nucleated red blood cells have been reported . 4 7. Metabolic. Acidosis, hypoglycemia (hyperinsulinism), hypocalcemia (increased phosphate load, correction of metabolic acidosis), and hyponatremia/syndrome of inappropriate antidiuretic hormone secretion (SIADH). Diagnosis. careful history thorough physical examination appropriate laboratory studies Neurodiagnostic and neuroimaging studies EEG. may provide information on the severity of the asphyxia injury, and the type of EEG abnormality may be indicative of a specific pathologic variety. Identification of EEG abnormalities within the first hours after delivery may be helpful in selecting infants for treatment with neuroprotective agents. CT scan. The value of CT in the assessment of diffuse cortical neuronal injury is most apparent several weeks after severe asphyxial insults. It is of particular value in the identification of focal and multiple ischemic brain injury. During the first week after an insult, the striking, bilateral, diffuse hypodensity reflects marked cortical neuronal injury, with associated edema corresponding closely to the occurrence of maximum intracranial pressure. Ultrasonography is the method of choice for routine screening of the premature brain. It is of major value in the identification of IVH and necrosis of basal ganglia and thalamus. It is superior to CT in identifying both the acute and subacute-chronic manifestations of periventricular white matter injury. Its limitations in the first weeks of life include its inability to reliably identify mild injury, to visualize lesions that are peripherally located, and to distinguish between hemorrhagic and ischemic lesions in the cerebral parenchyma. MRI is the technique of choice for evaluation of hypoxic ischemic cerebral injury in term and premature newborns. Management: A. Optimal management is prevention. The first goal is to identify the fetus being subjected to or likely to experience hypoxic-ischemic insults with labor and delivery. B. Immediate resuscitation. Any newborn that is apneic at birth must be promptly resuscitated because it cannot be determined whether the infant is in primary or secondary apnea. 1. Maintenance of adequate ventilation. Use an assisted ventilatory rate to maintain physiologic levels of PCO2. Hypercarbia can further increase cerebral intracellular acidosis and impair cerebrovascular autoregulation, whereas hypocarbia (PaCO2 205 25 mm Hg) has been associated with PVL in preterm infants and late-onset sensorineural hearing loss in full-term infants. 2. Maintenance of adequate oxygenation (PaO2 40 in premature infants and PaO2 50 in term infants). Avoid hyperoxia , which may lead to additional brain injury from possible reduction in CBF and vaso-obliterative changes. 3. Maintenance of adequate perfusion. Maintain arterial blood pressure in the "normal" range for gestational age and weight. Volume expanders and inotropic support are often required. With the loss of cerebrovascular autoregulation, it is important to avoid systemic hypotension and hypertension. 4. Correct metabolic acidosis with cautious use of volume expanders. The primary objective is to sustain tissue perfusion. Perfuse or lose! Use bicarbonate only when cardiopulmonary resuscitation (CPR) is prolonged and the infant remains unresponsive. Bicarbonate administration may lead to hypercarbia and intracellular acidosis and increase lactate. 5. Maintain a normal serum glucose level (~75-100 mg/dL) to provide adequate substrate for brain metabolism. Avoid hyperglycemia to prevent hyperosmolality and a possible increase in brain lactate levels. 6. Control of seizures: a. Phenobarbital is the drug of choice. It is usually continued until the EEG is normal and there are no clinical seizures for 2 months. The benefit of prophylactic therapy remains controversial. High-dose phenobarbital (40 mg/kg) reduced the incidence of seizures and improved neurologic outcome at 3 years in term asphyxiated newborns . b. If seizures persist despite therapeutic phenobarbital levels--- diazepam, lorazepam, and phenytoin may be used 7. Prevention of cerebral edema. The cornerstone of prevention of serious brain swelling is avoidance of fluid overload. Maintain slight to moderate fluid restriction (eg, 60 mL/kg). If cerebral edema is severe, further restriction of fluid intake to 50 mL/kg is imposed. Observe the infant for SIADH. Glucocorticoids and osmotic agents are not recommended. C. Potential new therapies should aim at preventing delayed neuronal death once an asphyxial insult has occurred. 1. Magnesium has an inhibitory effect on excitation of the N-methyl-D- aspartate type of glutamate receptors and competitively blocks Ca2+ entry through voltagedependent Ca2+ channels during hypoxia. 2. Prevention of free radical formation a. Xanthine oxidase inhibitor. allopurinol reduced free radical formation and enhanced electrical brain activity in severely asphyxiated newborns. 6 b. Resuscitation with room air. 3. Excitatory amino acid antagonists. 4. Calcium channel blockers. 5. Inhibition of nitric oxide production. Increased plasma nitric oxide levels has been shown as a marker for severity of brain injury and poor neurologic outcome 6. Selective head cooling. Hypothermia is thought to protect the brain from injury by preventing the decline in high-energy phosphates. Phosphocreatine and adenosine triphosphate are maintained while cerebral lactate levels are reduced. Prognosis: Most survivors of perinatal asphyxia do not have major sequelae. A. Findings associated with increased risk of neurologic sequelae 1. Apgar score of 0-3 at 20 min of age. 2. Presence of multiorgan failure, particularly oliguria persisting beyond 24 h of life. 3. Severity of the neonatal neurologic syndrome. Severe HIE (Sarnat stage 3) carries a mortality rate of ~80%, and survivors often have multiple disabilities, including spastic CP, severe or profound mental retardation, cortical blindness, or seizure disorder . There is no permanent sequelae for mild HIE (Sarnat stage 1). Moderately affected (stage 2) patients have outcomes that vary with their overall clinical course and duration of their neurologic condition. Stage 2 beyond 5 days is a poorer prognostic sign. 4. Duration of neonatal neurologic abnormalities. Disappearance of neurologic abnormalities by 1-2 weeks and the ability to nipple feed normally is an excellent prognostic sign. 5. Presence of neonatal seizures, especially if they occur within the first 12 h after birth and are difficult to control. 6. An abnormal MRI obtained in the first 24-72 h is associated with a poor outcome, irrespective of birth variables. 7. Severity and duration of EEG abnormalities. Normal to mildly abnormal EEG patterns within the first days after delivery are significantly correlated with normal outcomes. moderately to severely abnormal EEG patterns are significantly related to abnormal outcomes . 8. Persistent abnormalities of brainstem function are generally incompatible with long-term survival. 9. Abnormal visual, auditory, or somatosensory evoked potentials persisting beyond day 7 of life. 7 10. Subsequent hearing is normal in most children who have suffered perinatal or postnatal asphyxia. 11. Microcephaly at 3 months of age is predictive of poor neurodevelopmental outcome 12. Decreased cerebral concentrations of phosphocreatine or ATP at birth on quantitative 31P MRI . 13. Elevated brain lactate levels ,elevated ratio of lactate to Nacetylaspartate and lactate to choline on proton MRS, and low CSF cyclic adenosine monophosphate (cAMP) levels. 14. Increased CBF on Doppler sonography in the first 3 days after birth . 15. Decreased cerebral resistive index on Doppler sonography . 16. The presence of optic atrophy is an indicator of poor visual outcome . Many children with postasphyxial CNS abnormalities have lower visual acuity scores and smaller visual fields. 8 Respiratory and cardiovascular effects during prolonged asphyxia 9 10