c3_02_lesson
advertisement

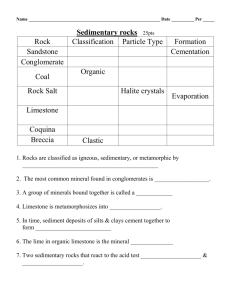
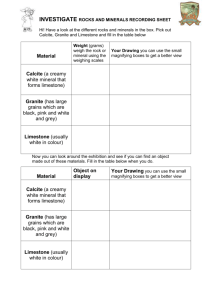
OCR 21st Century Science: C3 Chemicals in our lives – risks and benefits c3_02 Useful rocks Resources Student Book pages 174−175 Interactive Book: Matching pairs ‘Sedimentary rocks’ Homework pack c3_02 Files on Teacher Pack CD: c3_02_worksheet; c3_02_practical; c3_02_technician Samples of materials made using alkalis (particularly sodium carbonate); map of north-west England; pictures of early 19th century industries; samples of coal, limestone and rock salt; equipment for main practical Learning outcomes C3.1. 5 understand how processes such as mountain building, erosion, sedimentation, dissolving and evaporation have led to the formation of valuable resources found in England including coal, limestone and salt C3.1.6 understand how geologists study sedimentary rocks to find evidence of the conditions under which they were formed to include: a. fossils, b. shapes of water-borne grains compared to air-blown grains, c. presence of shell fragments; d. ripples from sea or river bottom C3.1.7 understand that chemical industries grow up where resources are available locally, e.g. salt, limestone and coal in north-west England Literacy focus: Extended writing. In this lesson students are learning to: explain how resources in the Earth were formed explain how geologists discover how rocks were formed explain how the development of the chemical industry depended on resources being available nearby Key vocabulary sediment sedimentary rock eroded dissolved evaporated Obstacles to learning This lesson requires a sense of the passage of geological time in order to explain the significance of the birth of the modern chemical industry. Note that students need to be familiar with the processes of the rock cycle, which they should have covered in KS3. Stimuli and starter suggestions Ask students to list the raw materials needed to make the materials that are all around them such as steel – iron ore, coal, limestone, air; bricks – clay; concrete – limestone, clay, sand; glass – sand, limestone, salt. Students will probably not know all these but if they get some they may see the pattern – most of the raw materials come from rocks. Learning activities worksheet c3_02 + practical c3_02 Low demand Show students samples of materials made using alkalis (particularly sodium carbonate) – e.g. glass, soap, oven cleaner, bleach, paper, washing soda. Explain that all these products (and many more) are manufactured by the chemical industry and that one of the places that it all started, early in the 19th century, was the north-west of England. There is a map on Student Book p. 174 but it would be useful to have an enlarged map of Britain to point out the area under discussion. Ask students why they think the industry started in this location – people (labour and markets) in the large towns (growing quickly because of the textile industry), transport (rivers, canals, seaports; not the M6!), and raw materials. Explain that the chemical industry needed three particular raw materials – coal, limestone and salt. All three were available close to the NW towns. Point out the locations on the map and ask why it was important to have all the raw materials nearby – ease of transport (roads difficult, no rail). Discuss how the raw materials were obtained by men quarrying and mining with simple tools and gunpowder. To summarise what they have learned, students could play the part of a 19th century industrialist keen to make a fortune with the repeal of the tax on salt in 1823, by writing a letter to a partner suggesting starting a chemical factory in the north-west, choosing a location and describing why they chose it. Teaching and learning notes: It would be useful to have pictures of the industrial towns, canals and ships, mines and quarries of the early 19th century to set the scene. Students do not need to recall the specific locations of the raw materials in the NW, just understand that the industry was established where they were available. For similar reasons chemical industries also grew up in Glasgow and on Tyneside. COLLINS NEW GCSE SCIENCE © HarperCollinsPublishers Ltd 2011 c3_02 Lesson continued Standard demand Students should investigate the processes that resulted in the formation of the mineral deposits. Student Book p. 174 outlines how coal, limestone and salt were formed. Note that although the deposits are close together in the NW, they formed at different times and under very different conditions because of the plate movements discussed in the previous lesson. Following the instructions on the practical sheet, students can model the processes of sedimentation that formed limestone, and of dissolution and evaporation that formed salt deposits. See also the technician sheet. Discuss the other processes of the rock cycle that resulted in the minerals being found where they are today – coal and salt buried underground under layers of other sedimentary rocks, and limestone exposed in the hills of the Peak District and suffering weathering and erosion. Students should compile this information into an illustration or presentation of the processes that formed the three raw materials. They can use the worksheet. Teaching and learning notes: During the discussion, assess students’ knowledge of the processes in the rock cycle and if necessary give them resources to help. Make sure that students understand how all the processes were involved in the formation of coal, limestone and salt. High demand Point out that students have learned and discussed the processes of formation of the rocks without looking for evidence that the ‘stories’ are correct. Discuss the evidence that geologists find. Samples of the three minerals and pictures of fossils in coal and limestone, of the sand grains in rock salt and the ripples in sediments (see Student Book Figures 4 and 5) would be useful. Discuss how these observations and others (weathering, erosion, transport and deposition) lead to the theories of geology. If there is time students should investigate the lives and work of James Hutton and Charles Lyle. Plenary suggestions Students could collaborate on preparing a mind-map of the lesson with ‘NW England Chemical Industry’ at the centre, and lines leading to reasons for the founding of the industry, the origins of the three raw materials and evidence for the processes of their formation. Student Book answers Q1 a) Coal, limestone, salt; b) Easy to transport to the factories. Q2 Sedimentation – limestone and coal; evaporation – salt; mountain building – limestone; dissolving – salt; erosion – limestone. Q3 Coal was formed in hot tropical climates; salt was formed when climates turned hot and dry. Q4 a) Contains small, smooth grains of wind-blown sand. b) Made up of shells of animals that lived in the sea and contains other fossil sea creatures; there may be ripples showing that the rock was formed on the bed of rivers or the sea. Practical sheet answers Method A Q1 Calcium carbonate. Q2 Build their shells. Q3 Sink into the sediment; form fossils in the rock. Q4 Deposited at the bottom of the sea. Q5 It is weathered and eroded. Method B Q1 a) Salt is dissolving; b) Rainwater trickling through the rocks would dissolve the salts. Q2 The Sun Q3 Salt; probably not pure; it contains some tiny particles of sand that were suspended in the water. Q4 Blown by the wind. Worksheet answers Activity 1 (Low demand) Q1 Coal – burning in steam engines; limestone – building and farming; salt – flavouring and preserving food. Activity 2 (Standard demand) Q1 Rock cycle diagram; coal – sedimentation and compression; limestone – sedimentation, compression, uplift, weathering, erosion; salt – dissolving, evaporation, compression. Q2 Moved through different climates; which resulted in different rocks being laid down. Activity 3 (High demand) Q1 Contains small, rounded grains. Q2 Waves when the sediment lay at the bottom of a river or sea. Q3 The age of the rock; the conditions in which the rock formed; the climate at the time. COLLINS NEW GCSE SCIENCE © HarperCollinsPublishers Ltd 2011