Supplementary Materials and Methods
advertisement

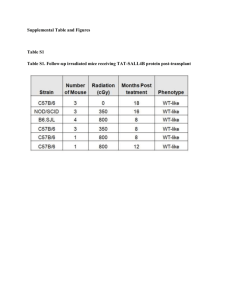
Supplementary Materials and Methods Animals and Treatment Groups The mice were anesthetized with intraperitoneal ketamine hydrochloride (60 mg/kg) and atropine sulfate (0.025mg/kg), and given either intranasal porcine pancreatic elastase (100 U/kg) (Sigma-Aldrich) in 20 µl of sterile saline solution (elastase-induced emphysema) or intranasal saline alone (control). Three weeks after elastase administration, 5 animals were sacrificed from each of these groups. Additional treatments were administered to four subgroups from the elastaseinduced emphysema or control animals (N 5 animals per each subgroup). In a first subgroup, after the initial elastase treatment, intranasal recombinant HGF (12.5 µg/dose in 20 µl solution) was given twice/week for 1, 2 or 4 weeks. In a second subgroup, also initially elastase-treated, intranasal saline was given twice/week for 1, 2 or 4 weeks. In a third subgroup, which had initially received saline, intranasal recombinant HGF was given twice/week for 1, 2 or 4 weeks. In a fourth subgroup, also initially saline-treated, intranasal saline was given twice/week for 1, 2 or 4 weeks. Animals in these subgroups were sacrificed after these secondary treatments. The elastase/HGF treatment protocol was repeated three times with wild type C57BL/6J mice: once for histopathology, once for lung mechanics measurements and once for protein and serum collections. An additional series was done with mice transplanted with bone marrow from GFP-transgenic mice for identification of the role of bone marrowderived cells (7 animals/group C, D and E and 5 animals/all other groups in each time). 1 In a preliminary study using blue dye, we confirmed that a liquid volume of 20 µl achieves near homogenous distribution to all lobes of both lungs when given by both the intranasal and intra-tracheal instillation techniques. As the intranasal route is less invasive and more physiological, we used it for both elastase and HGF administrations. Lung histopathology and quantitation of emphysema. Mean linear intercept (Lm) was determined by counting alveolar wall intersections with an array of test grid lines drawn horizontally on a printout of the digital image of each section and calculated according to the equation: Lm=2LT/Iw, where Iw is the number of times the test line intersected with an alveolar wall and LT is the length of the test line. Lung mechanics measurements Mice were terminally anesthetized with Ketamine (250mg/kg i.p.) then tracheostomized and the trachea were cannulated with a 20-gauge cannula connected to a computercontrolled small animal ventilator (flexiVent; Scireq, Montreal, PQ, Canada) [1]. Diaphragmatic paralysis was obtained by xylazine hydrochloride (12 mg/kg, i.p.) and mice were mechanically ventilated at 150 breaths/min with 6 ml/kg tidal volume. A positive end-expiratory pressure of 3 cmH2O was applied by submerging the expiratory line in water. Six-second sigh maneuvers to 3 times the tidal volume were performed three times to ensure similar volume history and establish a stable baseline respiratory system resistance and elastance. The compliance was determined by recording the relaxation pressures during inflation and deflation in 0.1-ml steps between 0 and 20 cm H2O. Pressures from the normalized compliance curves were extrapolated at 0.1-ml 2 increments and used to establish the mean static lung compliance and elastance in each group. Pressure volume curves were generated and automatically extrapolated and lung mechanics values calculated by the FelxiVent software. Lethal irradiation and reconstitution of bone marrow Adoptive transfer of bone marrow cells was performed as follow. Recipient C57BL/6 male mice were irradiated using two doses of 5 Gy, separated by 3 hours. Within 3 hours after irradiation, the recipient C57BL/6 mice were injected intravenously with donor bone marrow cells from GFP-transgenic mice (4 x 106 cells/200 µl media). Three weeks after transplantation, over 95% of the circulating white blood cells were GFP-positive, indicating that the recipient C57BL/6 mice had completely reconstituted with bone marrow cells of GFP mouse origin. FACS analysis Single cell suspensions were prepared from the right lungs of GFP-chimeric mice as follow. To remove most of the blood from lungs, animals were bled by transecting the abdominal aorta. The pulmonary vasculature was perfused with 10 ml of PBS at 25cmH2O through the right ventricle. One ml dispase I (2 unites/ml, Roche Diagnostics, Indianapolis, IN) was injected through the trachea, then lungs were incubated in a 37°C shaking incubator for 45 minutes in 10 ml of dispase, 1 ml of 0.001% DNAse (Sigma) and 1 ml of 2 μg/mL collagenase/dispase (Roche). The trachea and bronchi were removed, and lung minced and re-incubated for 10 minutes. This suspension was filtered through 100 μm mesh (BD Falcon, San Jose, CA), and centrifuged at 1000 rpm, 5 min at 3 4°C. Red blood cells were depleted by RBC lysis buffer (Sigma). Cells were resuspended in PBS at 1 x 106 cells/100 μL, and stained with anti-mouse Sca-1-APC, CD34-FITC, c-Kit-PE or CD45-PE and CD31-PE antibodies. Then, cells were analyzed by the FACSCalibur flow cytometer (Becton Dickinson). 4 Supplementary References: 1. Schuessler, TF, Bates, JH (1995) A computer-controlled research ventilator for small animals: design and evaluation. IEEE Trans Biomed Eng 42: 860-866. 5