Name - Ms Kim`s Biology Class
advertisement

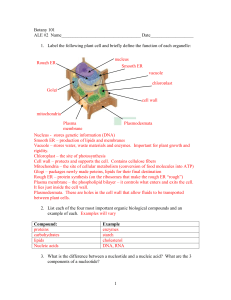
Name ____________________________________________ Date _________ Period ________ Osmosis Problems Label each of the situations as hypotonic, hypertonic, or isotonic: 1a. Cell _____Hypotonic___ 1b. Environment ___Hypertonic______ 2a. Cell _______Hypotonic______ 2b. Environment ______Hypertonic______ 3a. Cell _______Hypertonic_________ 3b. Environment ______Hypotonic_____ 4a. Cell ______Isotonic_______ 4b. Environment ______Isotonic_______ Read the following questions carefully. Be sure to answer all parts of each question. 5. If a cell contains 85% water and is placed into an environment which is 50% water. Is the cell hypotonic or hypertonic? In which direction will the water move, into the cell or out of the cell? Environment = Hypertonic Solution Cell = Hypotonic Out of the cell 6. A cell contains 85% water and is placed into a solution, which contains 90% water. Is the solution in the environment hypotonic or hypertonic? In which direction will the water move? Solution/Environment = Hypotonic Move into the cell 7. If a cell, which contains 90% water, is placed into an isotonic solution, what percentage of the solution is water? 90% 8. A cell contains 95% water; the environment outside the cell contains 45% solutes. Is the cell in a hypotonic or hypertonic environment? Hypertonic Solution 9. A cell containing 25% solutes is placed into a solution, which contains 35% solutes. Which direction will the water move, into the cell or out of the cell? Solution = Hypertonic solution Move out of the cell 10. A plant cell containing 95% water is placed into a 10% salt solution. Is the salt solution hypotonic or hypertonic? Which direction will the water move? Solution = hypertonic solution Out of the cell 11. If water leaves a cell when placed into a solution, is the cell hypotonic or hypertonic The cell is hypotonic The environment is hypertonic 12. What types of molecules have difficulty crossing the plasma membrane? Why? Polar (charged) molecules because it hates nonpolar and the cell membrane is nonpolar. Large molecules because it is too big to fit. 13. What is meant by a concentration gradient? The difference in concentration of a substance from one location to another. 14. A cell whose internal osmotic concentration is 0.3 osmoles/liter is placed in a solution that is 0.5 osmoles/liter. The solution is ____Hypertonic____ relative to the cell. 15. A cell is placed in a solution and swells. The solution is __hypotonic___ relative to the cell. 16. DIAGRAM BELOW: The solutions in the two arms of this U-tube are separated by a membrane that is permeable to water and glucose but not to sucrose. Side A is filled with a solution of 2.0 M sucrose and 1.0 M glucose. Side B is filled with a solution of 1.0 M sucrose and 2.0 M glucose. A. Initially, the solution in side A is _isotonic_ with respect to that in side B. B. After the system reaches equilibrium, describe what changes are observed. Glucose will move from side B to side A. There will be 2.0 M sucrose and 1.5 M glucose on side A. There will be 1.0 M sucrose and 1.5 M glucose on side B. Side B has more water, therefore, water will move from side B to side A and Side A will rise. 17. A semipermeable sac, containing 4% NaCl, 9% glucose, and 10% albumin, is suspended in a solution with the following composition: 10% NaCl, 10% glucose, and 40% albumin. Assume the sac is permeable to all substances except albumin. Use the key provided to determine how each substance will move. A. Moves into the sac ___B___ Glucose B. Moves out of the sac C. Does not move ___C___ Albumin ___B___ Water ____A__ NaCl 18. The diagrams below show three microscopic fields (A-C) containing red blood cells. Arrows indicate the direction of net water movement. Answer the following questions. a. b. c. d. e. Which microscopic field shows a hypertonic environment?___A____ Which microscopic field shows a cell hypertonic to its environment? ____C_______ Which microscopic field shows an isotonic environment?____B____ Describe what is happening to the cells in Field C: The environment has more water, therefore, the water is moving from high concentration of water to low concentration of water INTO the cell and the cell is going through cytolosis (swelling). Why is this happening? 19. Examine the drawing below. The tube, filled with 10% sucrose solution, was placed in a 5% sucrose solution. The membrane covering the bottom of the tube is impermeable to sucrose. a. In what direction does water move? ____Into the tube_____ b. Explain why in terms of osmosis: High concentration of water outside is moving into the tube that has low concentration of water. c. Are the tube contents hypertonic, hypotonic, or isotonic? ____Hypertonic____ d. Is the solution outside the tube hypertonic, hypotonic, or isotonic? __Hypotonic_____ 20. An artificial cell with an aqueous solution enclosed in a selectively permeable membrane has just been placed in an environment containing a different solution. The membrane is permeable to water and to the simple sugars glucose and fructose, but is completely impermeable to sucrose. Use this information and the diagram at the right to answer the questions that follow. a. Which solute(s) will exhibit a net diffusion into the cell? _______Fructose______ b. Which solute(s) will exhibit a net diffusion out of the cell? _______Glucose__________ c. In which direction will there be a net osmotic movement of water? ______Into the cell__________ d. After the cell is placed into the environment, which of the following changes would occur? (Check the correct one.) ________The artificial cell would be become flaccid. ___X__The artificial cell would become more turgid. _______There would be no net change in the cell. 21. During diffusion, when the concentration of molecules on both sides of a membrane is the same, the molecules will a. move across the membrane to the outside of the cell. b. stop moving across the membrane. c. continue to move across the membrane in both directions. d. move across the membrane to the inside of the cell. 22. The cell membrane is impermeable to sucrose. When the concentration of sucrose is higher on in the inside of the cell, the sucrose will a. move across the membrane to the outside of the cell. b. stop moving across the membrane. c. continue to move across the membrane in both directions. d. move across the membrane to the inside of the cell. 23. During osmosis, the cell is in an isotonic solution. The water will a. move across the membrane to the outside of the cell. b. stop moving across the membrane. c. continue to move across the membrane in both directions. d. move across the membrane to the inside of the cell. 24. An animal cell that is surrounded by fresh water will burst because the osmotic pressure causes a. water to move into the cell. b. solutes to move into the cell. c. water to move out of the cell. d. solutes to move out of the cell. 25. Diffusion occurs because a. molecules are attracted to one another. b. molecules constantly move and collide with each other. c. cellular energy forces molecules to collide with each other. d. cellular energy pumps molecules across the cell membrane.